For millions of Americans, taking medication is a routine and necessary part of maintaining health, but if you’ve watched drug advertisements on TV, you’re familiar with the litany of possible side effects. Many of us have experienced some – perhaps drowsiness from an antihistamine, nausea after one of the new GLP-1 weight-loss shots, or an episode of low blood pressure from a hypertension drug. For some people, a prescribed drug can trigger a severe, even life-threatening, reaction.
These adverse drug reactions (ADRs) are dangerous to patients and place a massive financial burden on healthcare systems. They lead to increased healthcare costs through emergency room visits, hospital admissions, longer hospital stays, and the need for additional treatments to manage the reactions. In the U.S., the costs may be as much as $30.1 billion annually.
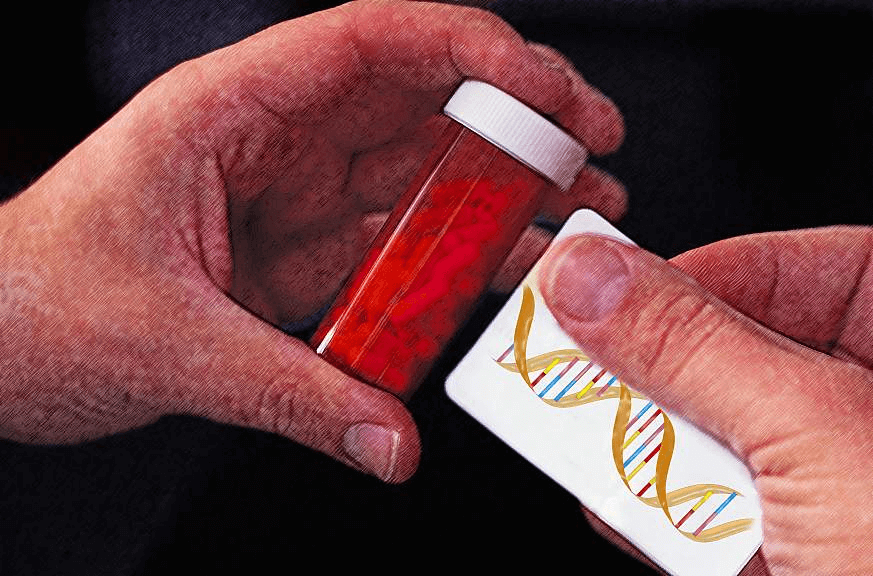
Now, imagine walking into a pharmacy and knowing that the medication you’re picking up has been chosen—and dosed—specifically for you, based on your DNA. And it won’t break your bank. That’s no longer science fiction. Thanks to rapid advances in genetic testing, tailoring medicines to an individual’s genetic profile is now a clinical reality.
By using a patient’s genetic information, doctors can predict in advance whether a drug will be effective, how it should be dosed, or whether it’s likely to cause harm. Instead of trial-and-error prescribing, clinicians can choose the right drug at the right dose the first time. One of its most powerful and immediate uses will be the prevention of harmful drug side effects, especially in patients taking psychiatric and cardiovascular medications.
A groundbreaking study from British researchers underscores just how transformative this approach could be. The researchers found that genetic testing for just three specific genes before a prescription is even written could prevent up to 75% of serious adverse drug reactions (ADRs). The research, published in PLOS Medicine, highlights how pharmacogenomic (PGx) testing—screening several of patients’ genes for how they respond to drugs — could not only make prescribing far safer but also reduce healthcare costs and prevent unnecessary hospitalizations.
Genetic clues behind adverse drug reactions
Not everyone processes medication in the same way. Our bodies metabolize drugs using enzymes, which are controlled by our genes. Variations in these genes – and, therefore, in the enzymes they express — can make a person metabolize a drug too quickly or too slowly, leading to dangerous side effects.
The study analyzed over 1.3 million ADR reports collected by the Yellow Card scheme, the U.K.’s official system for tracking medication side effects, and the findings were striking: Nine percent of ADRs were linked to drugs that interact with three key genes — CYP2C19, CYP2D6, and SLCO1B1 – that play a crucial role in how the body processes many commonly prescribed medications. Those ADRs were, therefore, considered to be “mitigatable,” or avoidable, by pre-administration genetic testing.
The researchers also found that certain classes of drugs were disproportionately linked to ADRs that could be prevented with genetic testing:
- Psychiatric Medications: Accounting for 47% of all genetically linked ADRs, these include antidepressants and antipsychotics, which can cause severe side effects in people with specific genetic variants.
- Cardiovascular Medications: Responsible for 24% of preventable ADRs, these drugs are commonly used for heart conditions and blood pressure management.
This research could lead to simple and effective tests. Genetic testing for CYP2C19, CYP2D6, and SLCO1B1 could allow doctors to personalize prescriptions and adjust drug choices and dosages based on a patient’s genetic makeup. This approach is neither theoretical nor completely new — previous research, including the international PREPARE trial, the results of which were reported in 2023, had already shown that some PGx testing can reduce ADRs by approximately 30% when incorporated into routine prescribing.
There are disparities in drug metabolism across different ethnic and racial populations. Any therapies that result from these findings could be particularly beneficial to people of African or Asian ancestry who appear to be at higher risk for some ADRs. Unfortunately, ethnic data were not available in the Yellow Card reports (which in the UK collects information on suspected safety concerns involving healthcare products, like a side effect with a medicine) but based on known genetic differences, PGx testing could disproportionately benefit these underrepresented groups and help reduce health inequalities.in drug metabolism across different ethnic and racial populations. Any therapies that result from these findings could be particularly beneficial to people of African or Asian ancestry who appear to be at higher risk for some ADRs. Unfortunately, ethnic data were not available in the Yellow Card reports [what are the Yellow Card reports?], but based on known genetic differences, PGx testing could disproportionately benefit these underrepresented groups and help reduce health inequalities.
Who would benefit the most?
 Credit: Pixabay/ Arek Socha
Credit: Pixabay/ Arek Socha
Why hasn’t pharmacogenomic testing become standard practice?
Despite its clear benefits, PGx testing is not yet widely used in healthcare systems like the U.K.’s National Health Service or in large Health Maintenance Organizations (HMOs) in the U.S. and elsewhere.
One major barrier is limited awareness and training among physicians. Many clinicians are unfamiliar with how to interpret PGx results or apply them to prescribing decisions, especially those who completedtheir education before genetic testing became clinically relevant. Compounding this is inconsistent insurance coverage. Many insurers still classify PGx testing as “non-essential” or “experimental,” particularly when not tied to a specific drug or diagnosis, discouraging both providers and patients from using it.
Another challenge is the lack of standardized guidelines. While strong evidence exists for certain gene-drug interactions—such as CYP2C19 and clopidogrel—many others lack clear clinical connections. This variability makes physicians hesitant to rely on test results. Additionally, integrating PGx into healthcare systems is often logistically difficult. Most hospitals lack the infrastructure to embed genetic data in electronic health records in a way that supports real-time alerts during prescribing. As a result, even when testing is done, the information may not be readily available or actionable at the point of care.
Scientific gaps also play a role. To date, much of the available pharmacogenomic research has been based on populations of European descent, making it less reliable for other ethnic groups. Broader inclusion is needed to ensure effective application of PGx across diverse populations. This study cracks open that door.
Financial considerations add further complexity: While PGx testing can reduce long-term costs by preventing adverse drug reactions, the upfront expenses of testing and system integration often deter adoption by healthcare systems focused on short-term budgets.
There are also regulatory and ethical concerns. Worries about genetic privacy, data security, and potential misuse—such as discrimination by insurers or employers based on test results—can make both patients and providers hesitant.
Finally, there’s a cultural resistance within medicine to change longstanding prescribing habits. Many physicians are reluctant to alter their workflows without strong institutional mandates or overwhelming evidence.
 Credit: NIH
Credit: NIH
Safer, personalized medicine is within reach
With landmark studies validating its life-saving potential, personalized medicine is no longer science fiction—it’s poised to become an achievable reality. Pharmacogenomic testing offers a clear path to safer, more effective treatment, particularly for patients on psychiatric and cardiovascular medications.
While the science behind pharmacogenomic testing is increasingly robust, its adoption into routine care is slowed by systemic, educational, financial, and cultural barriers. Overcoming these obstacles will require coordinated efforts in policy, medical education, infrastructure, and public awareness.
As healthcare systems evolve, integrating genetic testing into routine prescribing could become one of the most important innovations in modern medicine — saving lives, reducing costs, and making treatment more personalized.
Henry I. Miller is a physician and molecular biologist and the Glenn Swogger Distinguished Scholar at the Science Literacy Project. He was the founding director of the U.S. FDA’s Office of Biotechnology. Follow Henry on X @henryimiller