Setting and participants
In our prospective multicentre HCW cohort, participants were recruited from nine healthcare networks in Northern and Eastern Switzerland and followed since 08/2020. All hospital employees with or without patient contact, aged 16 years or older, were eligible for inclusion and enroled upon provision of informed consent. The study and protocol were approved by the Ethics Committee of Eastern Switzerland (#2020–00502).
Data collection
In October 2023, all new and ongoing participants provided or updated their baseline data (i.e., age, sex, health determinants, occupational and social life factors) and details on their history of SARS-CoV-2 infections (i.e., number and date of positive test results) and SARS-CoV-2 vaccinations (i.e., number, date and type). Participants were also asked to provide a serum sample tested for SARS-CoV-2 anti-spike (anti-S) and anti-nucleosid (anti-N) antibodies. Anti-S and anti-N were detected with the Roche Elecsys (Roche Diagnostics, Rotkreuz, Switzerland) electro-chemiluminescence immunoassay11. In weekly follow-up questionnaires between November 1st 2023 and April 30th 2024, participants indicated the presence of any of 22 respiratory, gastrointestinal and general symptoms (Supplementary Table 1) with an acute onset (new occurrence in the preceding 7 days) during the last 7 days, days of work absence attributable to symptoms, and documented any vaccination against SARS-CoV-2 or seasonal influenza including details on type of vaccine.
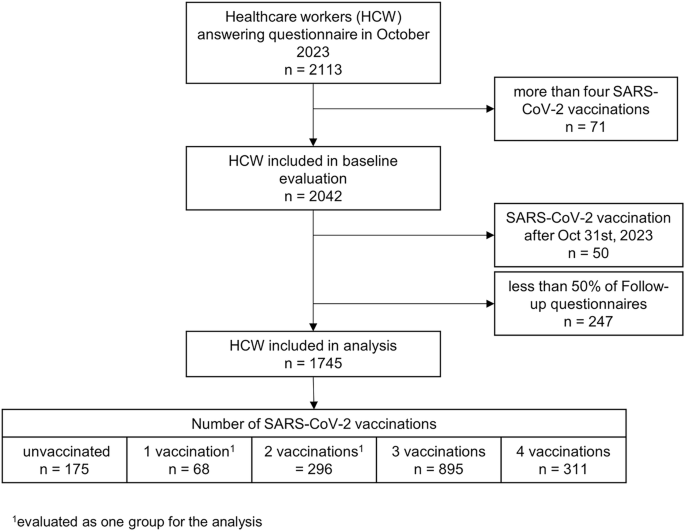
For this analysis, we included only those 1745 (87.6%) participants who provided at least 50% of follow-up questionnaires (i.e., 13 or more). Furthermore, we excluded those reporting more than 4 vaccinations, as ≥5 doses were only recommended for highly selected populations by the Swiss government, and those receiving a SARS-CoV-2 vaccination during the follow-up period.
Predictors and outcomes
Vaccination status was used as main predictor and treated as categorical variable, as we assumed a non-linear effect of the number of vaccinations on the outcome. Participants were allocated to being unvaccinated; having received 1 or 2 SARS-CoV-2 vaccine doses (because after the first COVID-19 wave, people with one vaccine dose and documented infection were considered fully vaccinated), 3 vaccinations (majority with 1st booster); or 4 vaccinations (majority with 2nd booster). Of those with 4 vaccinations, 85.2% received the bivalent vaccine. Exact definitions of other predictors are shown in Supplementary Table 2.
ILI was chosen as main outcome as it can indicate SARS-CoV-2 activity during periods of high community transmission levels12,13 and testing for SARS-CoV-2 has been widely abolished in the post-pandemic phase. During the study period, 21.1% of tested individuals in Switzerland with ILI were SARS-CoV-2 positive and 20.0% were positive for influenza14. In accordance with the Centres for Disease Control and Prevention (CDC)15 and European Centre for Disease Prevention and Control (ECDC)16, ILI was defined as the occurrence of fever (≥38.0 °C) or feeling of feverishness AND a respiratory symptom (cough, sore throat, rhinitis or the loss of smell) AND an acute onset ≤7 days before respective reporting date. Sensitivity analyses were performed using two different case definitions, one being more lenient (acute onset of fever ≥ 38.0 °C or feeling of feverishness AND any other of the symptoms asked), one being more restrictive (fever ≥ 38.0 °C or feeling of feverishness AND ≥ 1 general symptom among fatigue, headache, and malaise AND ≥ 2 other symptoms). As secondary outcome, the number of workdays lost due to these symptoms was examined.
Statistical analyses
Baseline characteristics and outcomes by vaccination status were compared using two-sided Chi-square tests for categorical variables and two-sided Kruskal-Wallis test for continuous variables (assuming non-normal distribution).
To identify factors associated with number of ILI and workdays lost, uni- and multivariable regression analysis were performed using negative binomial models with number of answered follow-up questionnaires as offset term (complete case analysis). Incidence rate ratios (IRR) with corresponding 95% confidence intervals (CI) were calculated with adjustment for a priori defined confounders selected based on scientific knowledge of risk factors and associations found earlier in our cohort. These were age, sex, body mass index (BMI), smoking status, presence of any relevant comorbidity (i.e., cancer, immunosuppressive disorders, cardiovascular disease, lung disease), living with children under the age of 12, total number of positive SARS-CoV-2 swabs reported since the beginning of the pandemic and until October 2023, patient contact, and receipt of the seasonal influenza vaccine for 2023/2024 (Supplementary Table 2). Because of suspected multi-collinearity of vaccination status and time of last vaccination, two different models (model 1: without time of last vaccination; model 2: with time of last vaccination) were fitted. To account for influenza vaccination status as the potentially most important confounder, subgroup analysis for the outcome ILI was performed, including only HCW without seasonal influenza vaccination. To investigate the effect of bivalent vaccine formulations, an additional sensitivity analysis was performed, where participants receiving either 3 or 4 vaccinations were grouped together to avoid multi-collinearity (as those with bivalent vaccines were mostly those receiving 4 vaccinations).
To test the robustness of our findings and reduce potential confounding in investigating the effect of the SARS-CoV-2 vaccine, we performed inverse probability of treatment weighting (IPTW). First, propensity scores were calculated using generalized boosted model regression (mnps function from the R package ‘twang’) with the number of vaccines served as outcome and the following as independent variables which are known to influence the SARS-CoV-2 infection risk: age, sex, BMI, comorbidities, patient contact, children at home, previous positive swabs, and smoking status. To account for extreme propensity scores and improve robustness, the overlap weighting method17 was used to calculate the weights. Covariate balance after weighting was assessed using standardized mean differences (SMDs) with SMDs of less than 0.1 indicating sufficient balance. In the IPTW analysis, both negative binomial models were performed, which allowed for a more accurate estimation of the average treatment effect of receiving a certain number of vaccines on the number of ILIs. We used statistical software R (version 4.4.0) with the packages ‘tableone’, ‘nlme’, ‘MASS’ and ‘twang’ for the analyses. Statistical significance level was defined at α = 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.