Osborne, T. B., Mendel, L. B. & Ferry, E. L. The effect of retardation of growth upon the breeding period and duration of life of rats. Science 45, 294–295 (1917).
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J. Nutr. 10, 63–79 (1935).
Speakman, J. R., Mitchell, S. E. & Mazidi, M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp. Gerontol. 86, 28–38 (2016).
Shanley, D. P. & Kirkwood, T. B. Calorie restriction and aging: a life-history analysis. Evolution 54, 740–750 (2000).
Weindruch, R., Walford, R. L., Fligiel, S. & Guthrie, D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 116, 641–654 (1986).
Perkins, S. N., Hursting, S. D., Phang, J. M. & Haines, D. C. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wild-type mice. J. Invest. Dermatol. 111, 292–296 (1998).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009).
Colman, R. J. et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 5, 3557 (2014).
Pinney, D. O., Stephens, D. F. & Pope, L. S. Lifetime effects of winter supplemental feed level and age at first parturition on range beef cows. J. Anim. Sci. 34, 1067–1074 (1972).
Lawler, D. F. et al. Diet restriction and ageing in the dog: major observations over two decades. Br. J. Nutr. 99, 793–805 (2008).
McCay, C., Maynard, L., Sperling, G. & Barnes, L. L. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr. Rev. 33, 241–243 (1975).
Bagherniya, M., Butler, A. E., Barreto, G. E. & Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: a review of the literature. Ageing Res. Rev. 47, 183–197 (2018).
Fontana, L., Nehme, J. & Demaria, M. Caloric restriction and cellular senescence. Mech. Ageing Dev. 176, 19–23 (2018).
Radler, M. E., Wright, B. J., Walker, F. R., Hale, M. W. & Kent, S. Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience 285, 236–247 (2015).
Park, C. Y., Park, S., Kim, M. S., Kim, H. K. & Han, S. N. Effects of mild calorie restriction on lipid metabolism and inflammation in liver and adipose tissue. Biochem. Biophys. Res. Commun. 490, 636–642 (2017).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Most, J., Tosti, V., Redman, L. M. & Fontana, L. Calorie restriction in humans: an update. Ageing Res. Rev. 39, 36–45 (2017).
Cooper, T. M., Mockett, R. J., Sohal, B. H., Sohal, R. S. & Orr, W. C. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 18, 1591–1593 (2004).
Mockett, R. J., Cooper, T. M., Orr, W. C. & Sohal, R. S. Effects of caloric restriction are species-specific. Biogerontology 7, 157–160 (2006).
Liao, C. Y., Rikke, B. A., Johnson, T. E., Diaz, V. & Nelson, J. F. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95 (2010).
Burnett, C. et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485 (2011).
Forster, M. J., Morris, P. & Sohal, R. S. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 17, 690–692 (2003).
Speakman, J. R. & Mitchell, S. E. Caloric restriction. Mol. Asp. Med. 32, 159–221 (2011).
Di Francesco, A. et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 634, 684–692 (2024).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span-from yeast to humans. Science 328, 321–326 (2010).
Green, C. L. et al. The effects of graded levels of calorie restriction: XVI. Metabolomic changes in the cerebellum indicate activation of hypothalamocerebellar connections driven by hunger responses. J. Gerontol. A Biol. Sci. Med. Sci. 76, 601–610 (2021).
Dirks, A. J. & Leeuwenburgh, C. Caloric restriction in humans: potential pitfalls and health concerns. Mech. Ageing Dev. 127, 1–7 (2006).
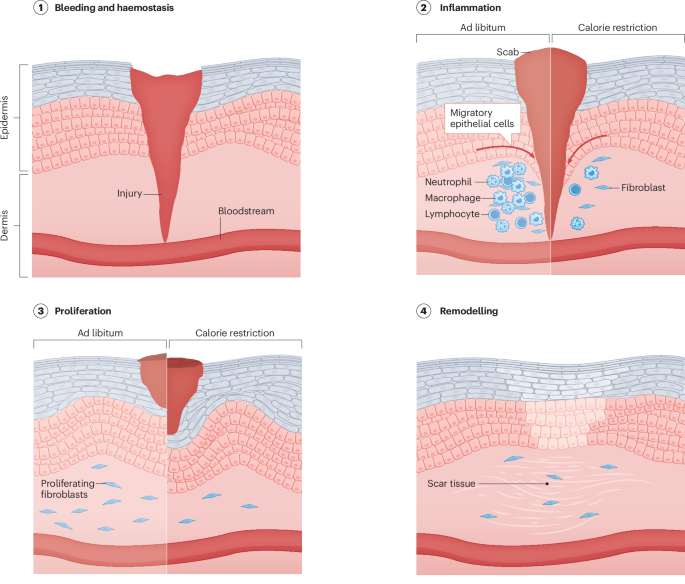
Guo, S. & Dipietro, L. A. Factors affecting wound healing. J. Dent. Res. 89, 219–229 (2010).
Gosain, A. & DiPietro, L. A. Aging and wound healing. World J. Surg. 28, 321–326 (2004).
Swift, M. E., Burns, A. L., Gray, K. L. & DiPietro, L. A. Age-related alterations in the inflammatory response to dermal injury. J. Invest. Dermatol. 117, 1027–1035 (2001).
Swift, M. E., Kleinman, H. K. & DiPietro, L. A. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Invest. 79, 1479–1487 (1999).
Barchitta, M. et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 20, 1119 (2019).
Reiser, K., McGee, C., Rucker, R. & McDonald, R. Effects of aging and caloric restriction on extracellular matrix biosynthesis in a model of injury repair in rats. J. Gerontol. A Biol. Sci. Med. Sci. 50a, B40–B47 (1995).
Harrison, D. E. & Archer, J. R. Genetic differences in effects of food restriction on aging in mice. J. Nutr. 117, 376–382 (1987).
Reed, M. J. et al. Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice. Mech. Ageing Dev. 89, 21–43 (1996).
Hunt, N. D. et al. Effect of calorie restriction and refeeding on skin wound healing in the rat. Age 34, 1453–1458 (2012).
Wolf, N. S. in The Comparative Biology of Aging (ed. Wolf, N. S.) 97–122 (Springer, 2009).
Hsieh, E. A., Chai, C. M. & Hellerstein, M. K. Effects of caloric restriction on cell proliferation in several tissues in mice: role of intermittent feeding. Am. J. Physiol. Endocrinol. Metab. 288, E965–E972 (2005).
Iwamura, H. et al. Caloric restriction reduces basal cell proliferation and results in the deterioration of neuroepithelial regeneration following olfactotoxic mucosal damage in mouse olfactory mucosa. Cell Tissue Res. 378, 175–193 (2019).
Roth, G. S. et al. Effect of age and caloric restriction on cutaneous wound closure in rats and monkeys. J. Gerontol. A Biol. Sci. Med. Sci. 52, B98–B102 (1997).
Weindruch, R. H., Kristie, J. A., Cheney, K. E. & Walford, R. L. Influence of controlled dietary restriction on immunologic function and aging. Fed. Proc. 38, 2007–2016 (1979).
Carrillo, A. E. & Flouris, A. D. Caloric restriction and longevity: effects of reduced body temperature. Ageing Res. Rev. 10, 153–162 (2011).
Lane, M. A. et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc. Natl Acad. Sci. USA 93, 4159–4164 (1996).
Mitchell, S. E. et al. The effects of graded levels of calorie restriction: III. Impact of short term calorie and protein restriction on mean daily body temperature and torpor use in the C57BL/6 mouse. Oncotarget 6, 18314–18337 (2015).
Forsum, E., Hillman, P. E. & Nesheim, M. C. Effect of energy restriction on total heat production, basal metabolic rate, and specific dynamic action of food in rats. J. Nutr. 111, 1691–1697 (1981).
Hambly, C. & Speakman, J. R. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes. Res. 13, 1548–1557 (2005).
Rikke, B. A. et al. Strain variation in the response of body temperature to dietary restriction. Mech. Ageing Dev. 124, 663–678 (2003).
Turturro, A. & Hart, R. W. Longevity-assurance mechanisms and caloric restriction. Ann. N. Y. Acad. Sci. 621, 363–372 (1991).
Hunter, W. S., Croson, W. B., Bartke, A., Gentry, M. V. & Meliska, C. J. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol. Behav. 67, 433–437 (1999).
Conti, B. et al. Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828 (2006).
Zhao, Z. et al. Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nat. Metab. 4, 320–326 (2022).
Hauck, S. J., Hunter, W. S., Danilovich, N., Kopchick, J. J. & Bartke, A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp. Biol. Med. 226, 552–558 (2001).
Cintron-Colon, R., Shankar, K., Sanchez-Alavez, M. & Conti, B. Gonadal hormones influence core body temperature during calorie restriction. Temperature 6, 158–168 (2019).
Johnstone, A. M., Murison, S. D., Duncan, J. S., Rance, K. A. & Speakman, J. R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 82, 941–948 (2005).
Roth, G. S. et al. Effects of dietary caloric restriction and aging on thyroid hormones of rhesus monkeys. Horm. Metab. Res. 34, 378–382 (2002).
Fontana, L., Klein, S., Holloszy, J. O. & Premachandra, B. N. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 91, 3232–3235 (2006).
Cintron-Colon, R. et al. Activation of kappa opioid receptor regulates the hypothermic response to calorie restriction and limits body weight loss. Curr. Biol. 29, 4291–4299 (2019).
Sheng, Y. et al. Differential responses of white adipose tissue and brown adipose tissue to calorie restriction during aging. J. Gerontol. A Biol. Sci. Med. Sci. 76, 393–399 (2021).
Selman, C. et al. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech. Ageing Dev. 126, 783–793 (2005).
Duffy, P. H., Feuers, R. J. & Hart, R. W. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol. Int. 7, 291–303 (1990).
Hebebrand, J. et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol. Behav. 79, 25–37 (2003).
Duffy, P. H., Feuers, R., Nakamura, K. D., Leakey, J. & Hart, R. W. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol. Int. 7, 113–124 (1990).
Ahima, R. S. & Antwi, D. A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. North Am. 37, 811–823 (2008).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Myers, M. G. Jr. & Olson, D. P. Central nervous system control of metabolism. Nature 491, 357–363 (2012).
Schwartz, M. W., Woods, S. C., Porte, D. Jr., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Sohn, J. W. Network of hypothalamic neurons that control appetite. BMB Rep. 48, 229–233 (2015).
Bruning, J. C. & Fenselau, H. Integrative neurocircuits that control metabolism and food intake. Science 381, eabl7398 (2023).
De Solis, A. J. et al. Reciprocal activity of AgRP and POMC neurons governs coordinated control of feeding and metabolism. Nat. Metab. 6, 473–493 (2024).
Fenselau, H. et al. A rapidly acting glutamatergic ARC->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat. Neurosci. 20, 42–51 (2017).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Hambly, C., Mercer, J. G. & Speakman, J. R. Hunger does not diminish over time in mice under protracted caloric restriction. Rejuvenation Res. 10, 533–542 (2007).
Derous, D. et al. The effects of graded levels of calorie restriction: VI. Impact of short-term graded calorie restriction on transcriptomic responses of the hypothalamic hunger and circadian signaling pathways. Aging 8, 642–663 (2016).
Maclean, P. S., Bergouignan, A., Cornier, M. A. & Jackman, M. R. Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–R600 (2011).
Polidori, D., Sanghvi, A., Seeley, R. J. & Hall, K. D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity 24, 2289–2295 (2016).
Dorling, J. L. et al. Change in self-efficacy, eating behaviors and food cravings during two years of calorie restriction in humans without obesity. Appetite 143, 104397 (2019).
Kahathuduwa, C. N., Binks, M., Martin, C. K. & Dawson, J. A. Extended calorie restriction suppresses overall and specific food cravings: a systematic review and a meta-analysis. Obes. Rev. 18, 1122–1135 (2017).
Kraus, W. E. et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7, 673–683 (2019).
Huang, T. H. & Ables, G. P. Dietary restrictions, bone density, and bone quality. Ann. N. Y. Acad. Sci. 1363, 26–39 (2016).
Devlin, M. J. et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Min. Res. 25, 2078–2088 (2010).
Bartell, S. M. et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J. Bone Min. Res. 26, 1710–1720 (2011).
Reid, I. R., Baldock, P. A. & Cornish, J. Effects of leptin on the skeleton. Endocr. Rev. 39, 938–959 (2018).
Turner, R. T. et al. Peripheral leptin regulates bone formation. J. Bone Min. Res. 28, 22–34 (2013).
Baek, K., Barlow, A. A., Allen, M. R. & Bloomfield, S. A. Food restriction and simulated microgravity: effects on bone and serum leptin. J. Appl. Physiol. 104, 1086–1093 (2008).
Mitchell, S. E. et al. The effects of graded levels of calorie restriction: I. Impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget 6, 15902–15930 (2015).
Banu, J., Orhii, P. B., Okafor, M. C., Wang, L. & Kalu, D. N. Analysis of the effects of growth hormone, exercise and food restriction on cancellous bone in different bone sites in middle-aged female rats. Mech. Ageing Dev. 122, 849–864 (2001).
Maier, G. W. & Kreis, M. E. Limited nutritional energy supply differentially impairs growth and bone mineralization of the developing lumbar vertebrae in minipigs. Bone 36, 512–520 (2005).
Soltani, S., Hunter, G. R., Kazemi, A. & Shab-Bidar, S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 27, 2655–2671 (2016).
Veronese, N. & Reginster, J. Y. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin. Exp. Res. 31, 753–758 (2019).
Villareal, D. T. et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Min. Res. 31, 40–51 (2016).
Villareal, D. T. et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch. Intern. Med. 166, 2502–2510 (2006).
Villareal, D. T. et al. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell 10, 96–102 (2011).
Colman, R. J., Beasley, T. M., Allison, D. B. & Weindruch, R. Skeletal effects of long-term caloric restriction in rhesus monkeys. Age 34, 1133–1143 (2012).
Hawkins, J., Cifuentes, M., Pleshko, N. L., Ambia-Sobhan, H. & Shapses, S. A. Energy restriction is associated with lower bone mineral density of the tibia and femur in lean but not obese female rats. J. Nutr. 140, 31–37 (2010).
Kalu, D. N. et al. Lifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 rats. Mech. Ageing Dev. 26, 103–112 (1984).
Westerbeek, Z. W., Hepple, R. T. & Zernicke, R. F. Effects of aging and caloric restriction on bone structure and mechanical properties. J. Gerontol. A Biol. Sci. Med. Sci. 63, 1131–1136 (2008).
Pifferi, F. et al. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun. Biol. 1, 30 (2018).
Lupien, S. J. et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1, 69–73 (1998).
Patel, N. V. & Finch, C. E. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol. Aging 23, 707–717 (2002).
Ramos-Cabrer, P. et al. Reversible reduction in brain myelin content upon marathon running. Nat. Metab. https://doi.org/10.1038/s42255-025-01244-7 (2025).
Hedden, T. & Gabrieli, J. D. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96 (2004).
Picq, J. L., Aujard, F., Volk, A. & Dhenain, M. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol. Aging 33, 1096–1109 (2012).
Bond, N. W., Everitt, A. V. & Walton, J. Effects of dietary restriction on radial-arm maze performance and flavor memory in aged rats. Neurobiol. Aging 10, 27–30 (1989).
Martin, B., Mattson, M. P. & Maudsley, S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res. Rev. 5, 332–353 (2006).
Bellush, L. L., Wright, A. M., Walker, J. P., Kopchick, J. & Colvin, R. A. Caloric restriction and spatial learning in old mice. Physiol. Behav. 60, 541–547 (1996).
Pitsikas, N., Carli, M., Fidecka, S. & Algeri, S. Effect of life-long hypocaloric diet on age-related changes in motor and cognitive behavior in a rat population. Neurobiol. Aging 11, 417–423 (1990).
Markowska, A. L. & Savonenko, A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging 23, 75–86 (2002).
Markowska, A. L. Life-long diet restriction failed to retard cognitive aging in Fischer-344 rats. Neurobiol. Aging 20, 177–189 (1999).
Sarker, M. R. et al. Curcumin mimics the neurocognitive and anti-inflammatory effects of caloric restriction in a mouse model of midlife obesity. PLoS One 10, e0140431 (2015).
Yanai, S., Okaichi, Y. & Okaichi, H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol. Aging 25, 325–332 (2004).
Padamsey, Z., Katsanevaki, D., Dupuy, N. & Rochefort, N. L. Neocortex saves energy by reducing coding precision during food scarcity. Neuron 110, 280–296 (2022).
Morgan, J. F. Eating disorders and reproduction. Aust. N. Z. J. Obstet. Gynaecol. 39, 167–173 (1999).
Morgan, J. F., Lacey, J. H. & Reid, F. Anorexia nervosa: changes in sexuality during weight restoration. Psychosom. Med. 61, 541–545 (1999).
Johnston, S. L. et al. Having it all: historical energy intakes do not generate the anticipated trade-offs in fecundity. Proc. Biol. Sci. 273, 1369–1374 (2006).
Selesniemi, K., Lee, H. J. & Tilly, J. L. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 7, 622–629 (2008).
Isola, J. V. V. et al. Mild calorie restriction, but not 17α-estradiol, extends ovarian reserve and fertility in female mice. Exp. Gerontol. 159, 111669 (2022).
Mossa, F. et al. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88, 92 (2013).
Harrath, A. H., Alrezaki, A., Mansour, L., Alwasel, S. H. & Palomba, S. Food restriction during pregnancy and female offspring fertility: adverse effects of reprogrammed reproductive lifespan. J. Ovarian Res. 10, 77 (2017).
Zanini, B. M. et al. Calorie restriction during gestation affects ovarian reserve in offspring in the mouse. Reprod. Fertil. Dev. 32, 1338–1349 (2020).
Martin, C. K. et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern. Med. 176, 743–752 (2016).
Effros, R. B., Walford, R. L., Weindruch, R. & Mitcheltree, C. Influences of dietary restriction on immunity to influenza in aged mice. J. Gerontol. 46, B142–B147 (1991).
Peck, M. D., Babcock, G. F. & Alexander, J. W. The role of protein and calorie restriction in outcome from Salmonella infection in mice. J. Parenter. Enter. Nutr. 16, 561–565 (1992).
Hasegawa, A. et al. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J. Surg. Res. 174, 136–141 (2012).
MacDonald, L., Radler, M., Paolini, A. G. & Kent, S. Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R172–R184 (2011).
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Ritz, B. W., Aktan, I., Nogusa, S. & Gardner, E. M. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J. Nutr. 138, 2269–2275 (2008).
Gardner, E. M., Beli, E., Clinthorne, J. F. & Duriancik, D. M. Energy intake and response to infection with influenza. Annu. Rev. Nutr. 31, 353–367 (2011).
Clinthorne, J. F., Beli, E., Duriancik, D. M. & Gardner, E. M. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J. Immunol. 190, 712–722 (2013).
Gardner, E. M. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J. Gerontol. A Biol. Sci. Med. Sci. 60, 688–694 (2005).
Ikeda, S. et al. Dietary restriction impairs neutrophil exudation by reducing CD11b/CD18 expression and chemokine production. Arch. Surg. 136, 297–304 (2001).
Clinthorne, J. F., Adams, D. J., Fenton, J. I., Ritz, B. W. & Gardner, E. M. Short-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infection. J. Nutr. 140, 1495–1501 (2010).
Shi, H. N., Scott, M. E., Stevenson, M. M. & Koski, K. G. Energy restriction and zinc deficiency impair the functions of murine T cells and antigen-presenting cells during gastrointestinal nematode infection. J. Nutr. 128, 20–27 (1998).
Sun, D., Muthukumar, A. R., Lawrence, R. A. & Fernandes, G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin. Diagn. Lab. Immunol. 8, 1003–1011 (2001).
Kristan, D. M. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 6, 817–825 (2007).