Researchers in Germany have challenged a 200-year-old assumption and revealed that pressure and friction are not responsible for making ice slippery, contrary to what has long been taught in physics classrooms around the world.
Led by Martin Müser, PhD, a materials simulation professor at Saarland University, the team of physicists discovered that molecular dipoles are the true reason why humans, animals, and even machines lose their footing on ice.
The insight reportedly overturns a paradigm established nearly two centuries ago by popular British mathematician Lord Kelvin’s brother, James Thompson, who at the time proposed that pressure and friction contribute to ice melting alongside temperature.
“It turns out that neither pressure nor friction plays a particularly significant part in forming the thin liquid layer on ice,” Müser stated.
Rethinking a centuries-old theory
Müser and his colleagues, Achraf Atila, PhD, a computational material scientist, and Sergey Sukhomlinov, PhD, a postdoctoral physics researcher at the university, emphasized how for generations the common explanation for why ice becomes slippery has focused on pressure and friction.
They added that on icy winter pavements, it has long been believed that the combination of body weight and the warmth of shoe soles generates enough pressure to melt the surface, ultimately leading to slips and falls.
However, according to their newest research, it’s actually the interaction between molecular dipoles in the ice and those in the contacting surface, such as a shoe sole, that disrupts the ice’s structure and makes it slippery.
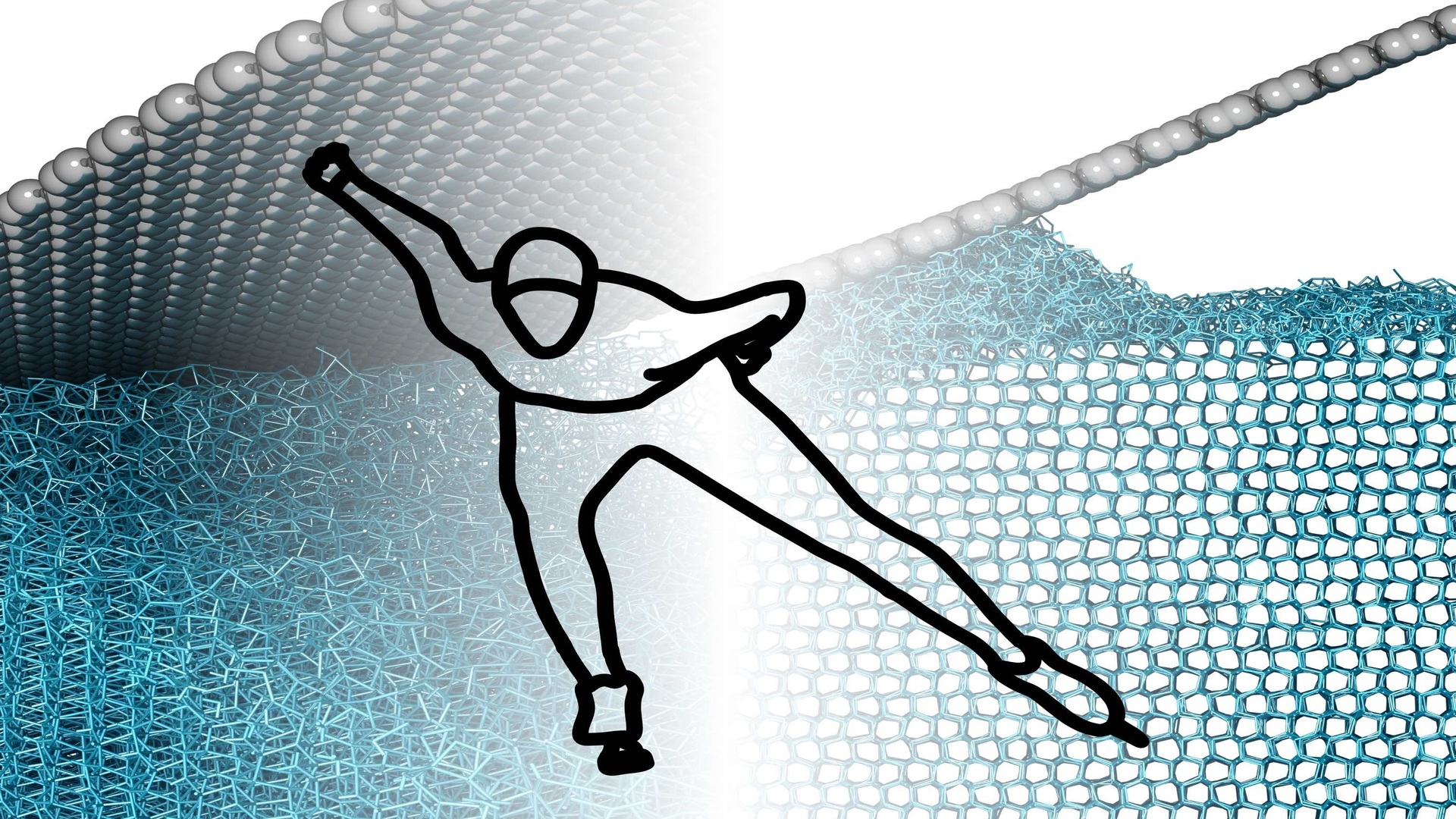 The illustration shows how contact with ice, whether through skis, skates, or shoes disrupts its orderly crystal structure. Credit: AG Müser
The illustration shows how contact with ice, whether through skis, skates, or shoes disrupts its orderly crystal structure. Credit: AG Müser
To challenge the long-held belief, the team used advanced computer simulations, which revealed that molecular dipoles, which occur due to the unequal sharing of electrons between atoms in a molecule, are the key drivers behind the formation of this slippery layer.
According to scientists, a molecular dipole arises when a molecule has partial positive and partial negative charge regions, giving the molecule an overall polarity that points in a specific direction.
Slippery even near absolute zero
The team took a closer look at how ice forms to understand the phenomenon. Below the freezing point, water molecules organize into a rigid crystal lattice, aligning in a highly ordered pattern that gives ice its solid and structured form.
When an individual steps onto the surface of ice, it’s not pressure or friction that causes it to become slippery, but rather the interaction between molecular dipoles in the shoe sole and those in the ice. This contact instantly disrupts the previously well-ordered crystal structure, making it unstable and slick.
“In three dimensions, these dipole-dipole interactions become ‘frustrated’,” Müser elaborated, referring to a physics concept where competing forces prevent a stable, ordered structure.
 Martin Müser, PhD, a materials simulation professor at Saarland University. Credit: Saarland University / Thorsten Mohr
Martin Müser, PhD, a materials simulation professor at Saarland University. Credit: Saarland University / Thorsten Mohr
At the microscopic level, this frustration at the ice-shoe interface disrupts the crystal lattice, turning it disordered, amorphous, and eventually liquid. What’s more in overturning Thompson’s theory, the team also debunked another widespread misconception.
“Until now, it was assumed that skiing below –40 degrees Celsius is impossible because it’s simply too cold for a thin lubricating liquid film to form beneath the skis,” Müser revealed in a press release. “That too, it turns out, is incorrect.”
He explained that dipole interactions persist even at extremely low temperatures. Remarkably, a liquid film still forms between ice and ski, even near absolute zero. The film becomes thicker than honey at such temperatures, hardly recognizable as water, and far too viscous for skiing. However, it still exists.
The study has been published in the journal Physical Review Letters.
