For media enquiries please contact Tim Mayo, Press Office, University of Birmingham, tel: +44 (0)7815 607 157.
Notes to editor:
University of Birmingham
The University of Birmingham is ranked amongst the world’s top 100 institutions. Its work brings people from across the world to Birmingham, including researchers, educators and more than 40,000 students from over 150 countries.
England’s first civic university, the University of Birmingham is proud to be rooted in of one of the most dynamic and diverse cities in the country. A member of the Russell Group and a founding member of the Universitas 21 global network of research universities, the University of Birmingham has been changing the way the world works for more than a century.
The University of Birmingham is committed to achieving operational net zero carbon. It is seeking to change society and the environment positively, and use its research and education to make a major global contribution to the UN Sustainable Development Goals. Find out at birmingham.ac.uk/sustainability.
The University of Birmingham is a founding member of Birmingham Health Partners (BHP), a strategic alliance which transcends organisational boundaries to rapidly translate healthcare research findings into new diagnostics, drugs and devices for patients. Birmingham Health Partners is a strategic alliance between nine organisations who collaborate to bring healthcare innovations through to clinical application:
- University of Birmingham
- University Hospitals Birmingham NHS Foundation Trust
- Birmingham Women’s and Children’s Hospitals NHS Foundation Trust
- Aston University
- The Royal Orthopaedic Hospital NHS Foundation Trust
- Sandwell and West Birmingham Hospitals NHS Trust
- Health Innovation West Midlands
- Birmingham and Solihull Mental Health NHS Foundation Trust
- Birmingham Community Healthcare NHS Foundation Trust
About the National Institute for Health and Care Research
The mission of the National Institute for Health and Care Research (NIHR) is to improve the health and wealth of the nation through research. We do this by:
- Funding high quality, timely research that benefits the NHS, public health and social care;
- Investing in world-class expertise, facilities and a skilled delivery workforce to translate discoveries into improved treatments and services;
- Partnering with patients, service users, carers and communities, improving the relevance, quality and impact of our research;
- Attracting, training and supporting the best researchers to tackle complex health and social care challenges;
- Collaborating with other public funders, charities and industry to help shape a cohesive and globally competitive research system;
- Funding applied global health research and training to meet the needs of the poorest people in low and middle income countries.
NIHR is funded by the Department of Health and Social Care. Its work in low and middle income countries is principally funded through UK international development funding from the UK government.
This study has been delivered through the National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
The NIHR Birmingham Biomedical Research Centre (BRC) is part of the NIHR and hosted by University Hospitals Birmingham NHS Foundation Trust (UHBFT) in partnership with the University of Birmingham (UoB). The BRC’s research programme focuses on inflammation and the diagnosis, prevention and treatment of its associated long-term illnesses.
About BEXMAB
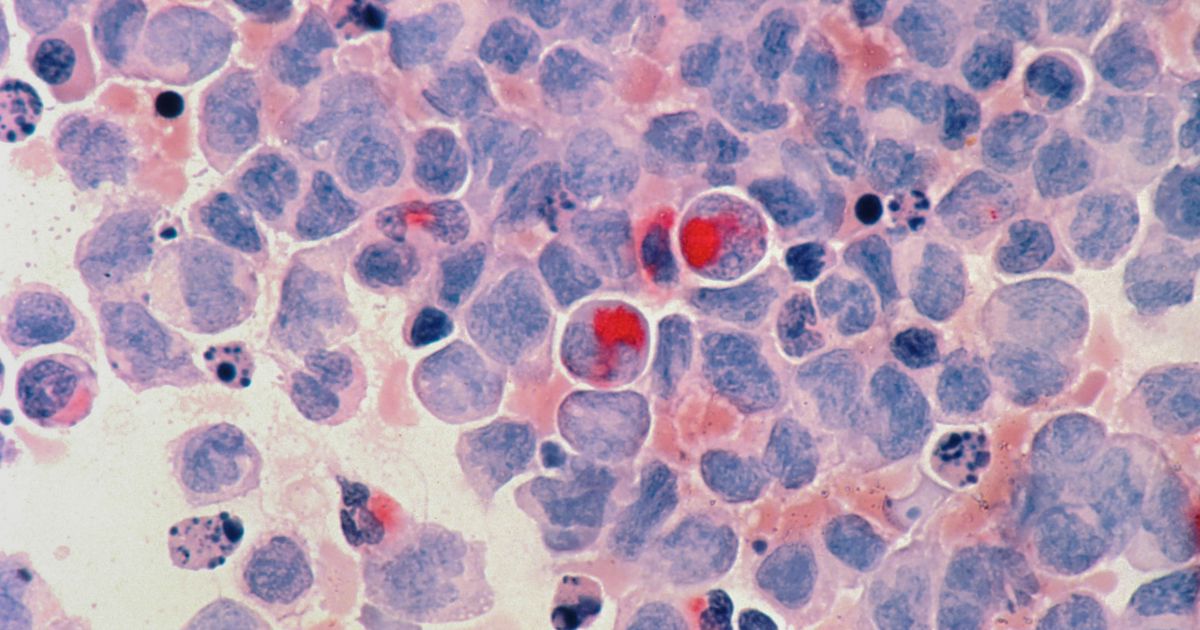
The BEXMAB study is an open-label Phase I/II clinical trial investigating bexmarilimab in combination with standard of care (SoC) in the aggressive hematological malignancies of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The primary objective is to determine the safety and tolerability of bexmarilimab in combination with SoC (azacitidine) treatment. Directly targeting Clever-1 could limit the replication capacity of cancer cells, increase antigen presentation, ignite an immune response, and allow current treatments to be more effective. Clever-1 is highly expressed in both AML and MDS and associated with therapy resistance, limited T cell activation and poor outcomes.
About bexmarilimab
Bexmarilimab is Faron’s wholly owned, investigational immunotherapy designed to overcome resistance to existing treatments and optimize clinical outcomes, by targeting myeloid cell function and igniting the immune system. Bexmarilimab binds to Clever-1, an immunosuppressive receptor found on macrophages leading to tumor growth and metastases (i.e. helps cancer evade the immune system). By targeting the Clever-1 receptor on macrophages, bexmarilimab alters the tumor microenvironment, reprogramming macrophages from an immunosuppressive (M2) state to an immunostimulatory (M1) one, upregulating interferon production and priming the immune system to attack tumors and sensitizing cancer cells to standard of care.
About Faron Pharmaceuticals Ltd
Faron (AIM: FARN, First North: FARON) is a global, clinical-stage biopharmaceutical company, focused on tackling cancers via novel immunotherapies. Its mission is to bring the promise of immunotherapy to a broader population by uncovering novel ways to control and harness the power of the immune system. The Company’s lead asset is bexmarilimab, a novel anti-Clever-1 humanized antibody, with the potential to remove immunosuppression of cancers through reprogramming myeloid cell function. Bexmarilimab is being investigated in Phase I/II clinical trials as a potential therapy for patients with hematological cancers in combination with other standard treatments. Further information is available at www.faron.com.