At this edition of the Heidelberg Laureate Forum, we welcomed back the first Young Researcher who came back as a Laureate. Amanda Randles attended the very first edition of the Heidelberg Laureate Forum, and in 2024, she was awarded the ACM Prize in Computing for her contributions to computational health.
Now, Randles came back to present some of her research on digital twins: models of the human body that promise to reshape how we approach healthcare.
 Amanda Randles. © HLFF / Kreutzer
Amanda Randles. © HLFF / Kreutzer
A Model of You
Digital twins are not a new concept. In fact, they have been used in some fields of engineering for some time. Sensors feed data into a detailed simulation that predicts wear, detects problems early, and guides repairs before disaster strikes. Randles envisions the same setup for humans., noting that this approach to medicine would be transformative.
Today, medicine is mostly reactive. We are waiting for problems to happen, then we try to treat them. With digital twins, the Laureate says, the aim is to flip that script and catch trouble early and treat it before it ever turns into a crisis.
Conceptually, the approach is similar to engineering. You feed the model as much data as possible about an individual’s body and you detect potential problems before they happen. For instance, you could detect cardiovascular problems before any clear symptoms emerge. Your doctor could test various treatment approaches or various surgery strategies and see which one should work better for your body. You could even do it to customize workouts and get the most out of your physical activity.
The problem, however, is that the human body is much more complex and difficult to emulate than a bridge. A digital twin is not just a static 3D model, it is a living computational model, constantly fed real-time data from thousands of sensors. To build it, Randles and her team fused together a dizzying array of data (the word “petabyte” came up a couple of times).
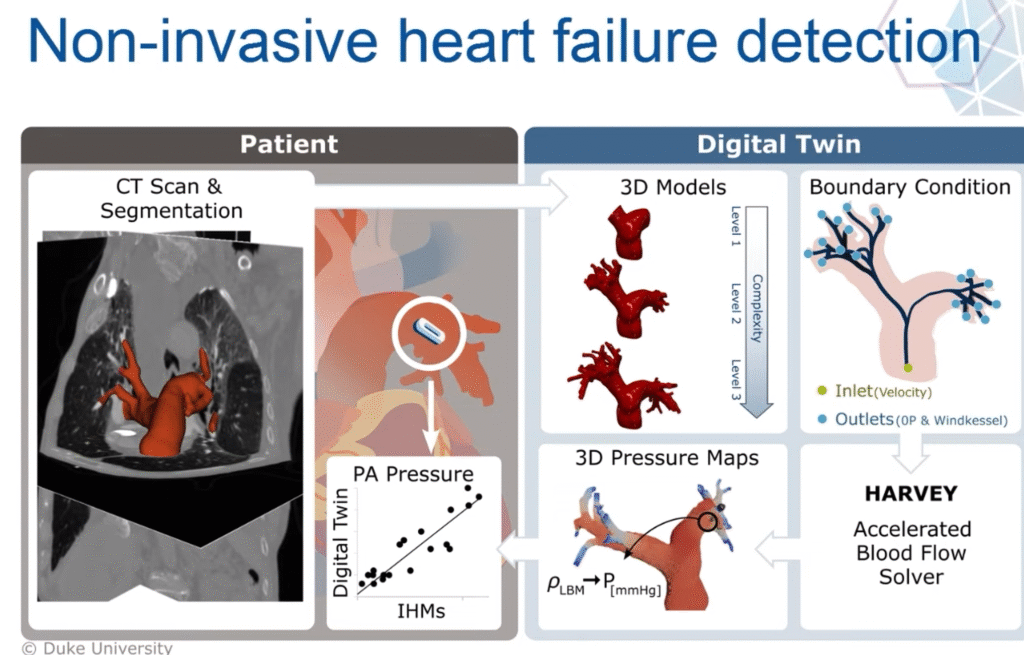
They focused on blood flow simulations, starting with medical imaging like MRI or CT scans. With these scans, they captured the unique geometry of people’s arteries. Then, they solve fluid dynamics equations to model blood flow inside this geometry. Randles focused on the circulatory system, building a tool called HARVEY, a high-performance blood flow simulator. Named after 17th-century physician William Harvey, who first described the circulation of blood, HARVEY uses advanced numerical methods to simulate blood at the cellular scale.
This is a gargantuan computational task.
The cardiovascular system alone contains more than 96,000 kilometers (60,000 miles) of blood vessels. And blood is not a simple fluid; it is a dynamic mix of cells, plasma, and proteins. Our bodies have 20 to 30 trillion red blood cells. To simulate just 30 seconds of blood flow in a single patient, the team used an entire supercomputer cluster for months.
Randles realized there had to be a better way.
Smarter Algorithms, More Data
 Slide from Randles’ lecture at the 12th HLF.
Slide from Randles’ lecture at the 12th HLF.
Along with her colleagues, she developed an algorithm that breaks down simulations into smaller, parallel pieces, allowing them to model weeks of blood flow using far less computational power. They take a model and then put these pieces back together. It is important to keep in mind that this is not purely a data-driven algorithm. There are hard, physical constraints regarding blood flow and fluid dynamics in HARVEY.
In one of Randles’ studies, they focused on coronary artery disease, a condition where fatty plaques narrow arteries and can trigger heart attacks. Traditionally, cardiologists measure something called fractional flow reserve (FFR), the ratio of blood pressure before and after a blockage to decide if a stent is necessary. But measuring FFR requires threading a wire through a patient’s artery, an invasive procedure.
Randles’ team built a digital model of each patient’s arteries and simulated the blood flow. Their calculations matched the invasive measurements within a few percentage points, close enough to guide treatment without sticking wires in anyone’s heart. Already, this shows how useful digital twins can be.
“What we’re trying to do is build this digital twin to not only be able to match these devices, but to be able to take it to where you can capture it as you’re moving about your daily life and be able to find other earlier symptoms.”
The model went further. It revealed a pattern of swirling blood, called vorticity, that predicted future complications better than FFR alone. With this, they were able to identify patients who looked fine by conventional measures but later developed problems, Randles said.
But the power of a twin isn’t just in diagnosing today’s disease. It’s in predicting tomorrow’s. To do that, Randles is drawing data from an increasingly abundant source: wearables. “We can say currently this pivotal shift in health care is driven by wearable sensors.”
These sensors, most often in the form of a smartwatch or a ring, offer a huge amount of data about sleep patterns, activity levels, heart rate, and many more. This opens the door for a range of personalized interventions, particularly when coupled with a digital twin. Doctors would no longer rely solely on population-based guidelines like “run 30 minutes a day” or “cut back on salt.” Instead, recommendations could be tuned to your physiology. Maybe a slow jog lowers your risk more than sprints. Maybe a small tweak in medication avoids a hospitalization entirely. The promise of wearable data, especially when coupled with digital twins, is to have these interventions custom-made for every individual.
It is an ambitious vision, and Randles’s ambition does not stop at organs and arteries. The ultimate understanding of disease happens at the level of individual cells. Her group is now pushing the boundaries of simulation to model the seemingly chaotic, microscopic world inside our blood vessels, capturing the behavior of single cells.
This Needs More Innovation
There is a reason Randles’ group involves includes mathematicians and computer scientists, not just doctors. This field needs breakthroughs in algorithms, hardware, and AI. Simulations must run faster, handle more data, and integrate wearable tech securely and fairly. AI models will need to predict outcomes accurately while explaining their reasoning. This is particularly important in medicine, where decisions cannot be black boxes.
She is also realistic about privacy and ethics. A digital twin would ultimately contain some of the most intimate data imaginable: your complete medical history, your genetic profile, your daily behavior. Making that secure and trustworthy is as important as making it accurate. This is all the more challenging as health data tends to be siloed.
“Our goal is to break down data silos. The way care is happening [..] you go to your cardiologist and your neurologist, and they don’t always connect as well as you’d want to. It’s not always seen as a whole patient so we’re trying to change this to a whole patient approach and really capture what’s happening in your entire lifespan.”
Pieces of this work are already in use. Non-invasive “digital FFR” is FDA approved in some contexts. Her lab’s vascular simulations are being tested in clinical collaborations. However, a reasonably complete digital twin is probably decades of work away. Still, Randles is convinced the payoff is worth it.
“In the future we won’t be ask if you have a digital twin but we’ll ask how your digital twin helped keep you healthy.”
Randles is also keen to point out the value of interdisciplinary events like the HLF. In a later conference, she noted how people like John Hopcroft and Vint Cerf were inspirational to her as a Young Researcher, and the HLF helped her think about machine learning “before we all started doing it.”
