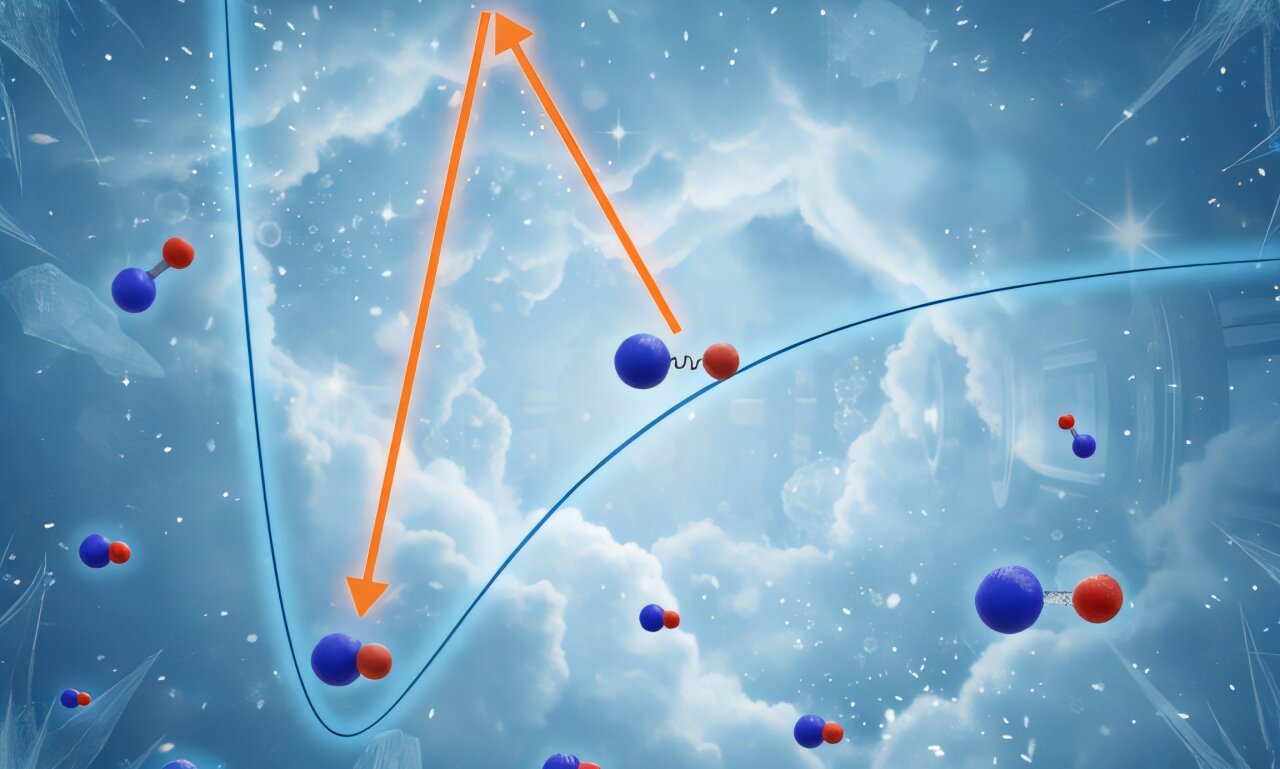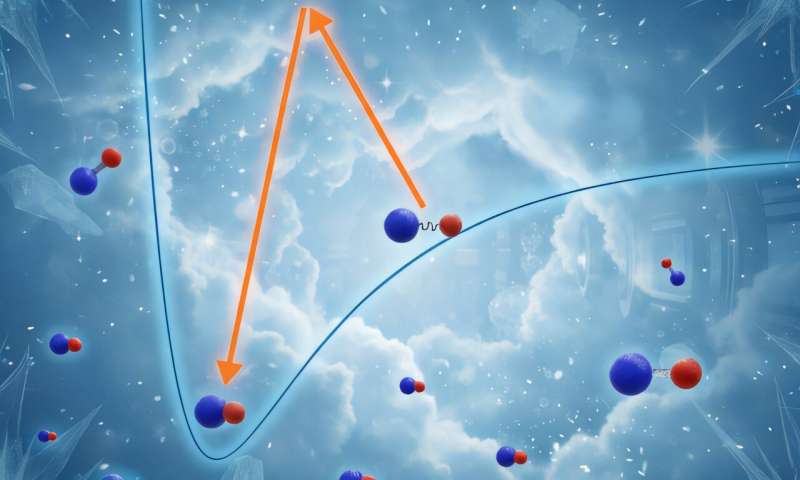
Weakly bound KCs molecules are transferred into what is known as their “absolute ground state.” Credit: Physical Review Letters (2025). DOI: 10.1103/gjzh-8dsb
Researchers from Hanns-Christoph Nägerl’s group have produced the world’s first ultracold KCs molecules in their absolute ground state. Starting by mixing clouds of potassium and cesium atoms cooled almost to absolute zero temperature, they were able to use a combination of magnetic fields and laser beams to associate pairs of freely moving atoms into chemically stable molecules.
The work is published in the journal Physical Review Letters.
Molecules can only be produced in chemical reactions, which always occur at unpredictable, random times. Higher temperatures make reactions faster, and sufficiently low temperatures may stop reactions from taking place altogether. These statements do not apply if chemistry is conducted by physicists.
In the last 20 years, several different types of molecules have been produced in gaseous mixtures at temperatures close to absolute zero, using methods that narrow the exact time at which the molecules are made to a few microseconds.
Until recently, KCs remained a gaping hole in the table of possible element combinations that have already been turned into molecules in this way.
Mixing is hard
In order to assemble molecules in a sufficiently controlled way, one needs to start with a mixture of ultracold atomic gases. Even though preparing such gases with a single element has become a standard experimental technique around the world, cooling two elements at the same time is a different story.
“Potassium and cesium were the last alkali elements to be cooled down to Bose-Einstein condensation on their own,” says Charly Beulenkamp, one of the lead authors of this study, “which indicates how difficult they are to control. Cooling them down at the same time is a challenge at an entirely different level.”
Luckily, thanks to the persistence of the team from Innsbruck, this challenge has finally been overcome.
Quantum leap of faith
The first step of assembling ultracold molecules is the so-called magneto-association, in which nearby atoms of different elements are turned into bound pairs by sweeping the external magnetic field across a resonant point. Such pairs are only bound very weakly, and could easily be broken apart—one could say that the atoms are now engaged, but not yet married.
To make these molecules chemically stable, they must be transferred into what is known as their absolute ground state: the state with the lowest energy among all possible states of a given molecule.
Transfers between different internal states of atoms or molecules can usually be performed using laser light tuned to an appropriate frequency, but in this case a direct transition is forbidden, requiring the use of a third, intermediate state as a pivoting point.
“Magneto-associated pairs and ground-state molecules are very different beings,” explains Krzysztof Zamarski, the other lead author of this work, “and turning one into the other is like pole-vaulting across a canyon. To be able to do this, one needs to find a supporting point for the pole, which has to be a tiny rock that is barely visible in the dark. Finding such a point is the main problem to be solved on the path towards producing ultracold molecules.”
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights.
Sign up for our free newsletter and get updates on breakthroughs,
innovations, and research that matter—daily or weekly.
Toy system for the study of materials
Quantum molecular synthesis can only produce a few thousand molecules at a time, so it is unlikely to replace conventional chemistry any time soon. However, it has many other exciting applications. One of the biggest questions in modern physics is why some materials exhibit certain exotic properties, such as superconductivity.
These phenomena are difficult to describe theoretically, because of the large number of particles involved, but it is also challenging to study them experimentally, due to the small length-scales on which they occur, as well as the imperfections that real materials suffer from. This is where ultracold gases, in particular gases of molecules, enter the stage.
Thanks to their big electric dipole moment, molecules composed of two different elements interact with each other across long distances, mimicking electrons in solid-state systems. At the same time, their low temperature makes it possible to trap them with laser light and manipulate them further using various techniques.
“Trapping molecules in a geometry that resembles real crystals gives us an opportunity to directly observe the quantum dynamics that govern exotic materials,” says Hanns-Christoph Nägerl from the Department of Experimental Physics at the University of Innsbruck, “which is the idea behind experimental quantum simulations.”
More information:
Krzysztof P. Zamarski et al, Spectroscopy and Ground-State Transfer of Ultracold Bosonic 39K133Cs MoleculesPhysical Review Letters (2025). DOI: 10.1103/gjzh-8dsb
Provided by
University of Innsbruck
Citation:
Ultracold potassium-cesium molecules assembled in absolute ground state (2025, November 17)
retrieved 17 November 2025
from https://phys.org/news/2025-11-ultracold-potassium-cesium-molecules-absolute.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.