Obe-cel is a type of CAR T-cell therapy developed in the UK and works by genetically modifying cells to enable the body’s own immune system to recognise and attack cancer.
The therapy, which only needs to be given once in a lifetime, was developed by Autolus, a University College London (UCL) spinout company.
The National Institute for Health and Care Excellence (Nice) has recommended obe-cel (also called Aucatzyl) in England for people aged 26 and over.
 Operators transferring patient starting material, obtained through a process called leukapheresis, to begin the manufacturing process at Autolus’ Nucleus site in Stevenage (Autolus/PA)
Operators transferring patient starting material, obtained through a process called leukapheresis, to begin the manufacturing process at Autolus’ Nucleus site in Stevenage (Autolus/PA)
The watchdog said the treatment could help more than 150 people over the next the three years who have relapsed or refractory B-cell acute lymphoblastic leukaemia (ALL), all with limited treatment options.
Obe-cel increases the likelihood of people going into remission and has fewer side-effects, meaning more people could benefit from it compared to other treatments.
Evidence from a clinical trial has shown high rates of remission in patients whose cancer has either returned (relapsed) after treatment or failed to respond to initial therapy (refractory).
A study of 94 people found that 77% given the treatment went into remission.
More than half of those were then showing no signs of detectable cancer after three and a half years.
Another CAR T-cell treatment is available for people aged 25 and under.
B-cell acute lymphoblastic leukaemia is a rare blood cancer, affecting fewer than five in 10,000 people in the UK.
Helen Knight, director of medicines evaluation, at Nice, said the treatment “offers real hope to people living with this rare and aggressive blood cancer”.
She added: “This drug has the potential to offer a more effective and less toxic alternative to standard treatments, with fewer side-effects.
“This could potentially be a life-saving drug, which will make a huge difference to people’s lives, including spending less time in hospital.”
 The therapy was developed by Autolus, a University College London spinout company (Autolus/PA)
The therapy was developed by Autolus, a University College London spinout company (Autolus/PA)
Dr Claire Roddie, UCL Hospital consultant haematologist and associate professor at the UCL Cancer Institute, said: “I am delighted to hear of Nice’s decision.
“Many more patients now stand to benefit from this CAR T-cell therapy on the NHS and we are still working to widen its application.
“Working on proving the safety and efficacy of this drug has brought together clinical and research teams from UCL and UCLH, with support from government and arms-length bodies like the National Institute for Health and Care Research (NIHR) and the Biomedical Research Centre as well as the pharmaceutical industry.
“The many people involved in this work can feel immensely proud of this achievement, which will help save the lives of many more patients.”
People receive two doses of the CAR T-cell treatment therapy intravenously, 10 days apart, with the treatment being delivered at selected specialist CAR T-cell centres across England.
Professor Peter Johnson, NHS national clinical director for cancer, said: “This cutting-edge therapy has shown real promise in trials and could give patients with this aggressive form of leukaemia a chance to live free from cancer for longer – and, for some, it could offer the hope of a cure.
“This ‘living medicine’ boosts a patient’s own immune system and then guides T-cells towards the cancer to kill it.
“It is fantastic to have another pioneering option available on the NHS, adding to our range of CAR T-cell therapies which are helping people with blood cancers live longer, healthier lives.”
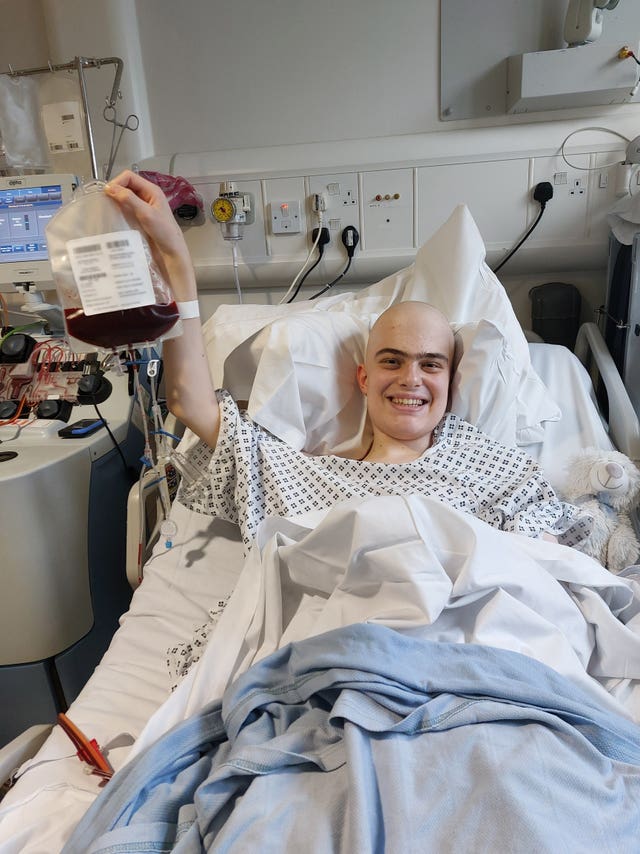 Harry Brown was treated with Obe-cel as part of a clinical trial in 2024 (Family handout/NHS England/PA)
Harry Brown was treated with Obe-cel as part of a clinical trial in 2024 (Family handout/NHS England/PA)
Harry, a 19-year-old student from Harrogate, was treated with Obe-cel as part of a clinical trial in 2024.
He said: “I feel so lucky to have had access to such a wonderous treatment.
“Not only did it work better than my doctors thought it would, it worked without many of the horrible side-effects you can get from other treatments.
“The biggest thing it offers is hope. When you’re facing a situation like mine, hope is the most valuable thing you can have.”
 Harry says the treatment offers hope (Family handout/NHS England/PA)
Harry says the treatment offers hope (Family handout/NHS England/PA)
Health minister, Ashley Dalton, said: “This pioneering treatment is excellent news for patients and their families, demonstrating how the NHS is at the forefront of medical innovation.”
Fiona Bride, interim chief commercial officer at NHS England, said the treatment represented “a success story that’s made in Britain”.
Fiona Hazell, chief executive at Leukaemia UK, said: “We are delighted that this therapy will be available on the NHS and this is a significant step forward in expanding treatment options for people living with leukaemia.”
