- Haiwang Yong
- Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA, US
August 20, 2025• Physics 18, 149
Scientists have used ultrashort x-ray pulses to directly observe the motion of electrons driving a chemical reaction.
I. Gabalski/Stanford University and SLAC National Accelerator Laboratory
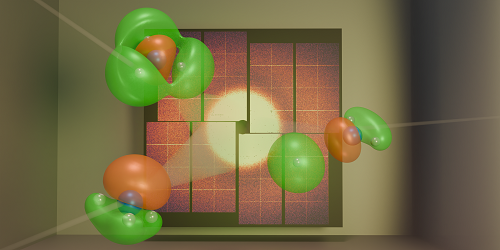
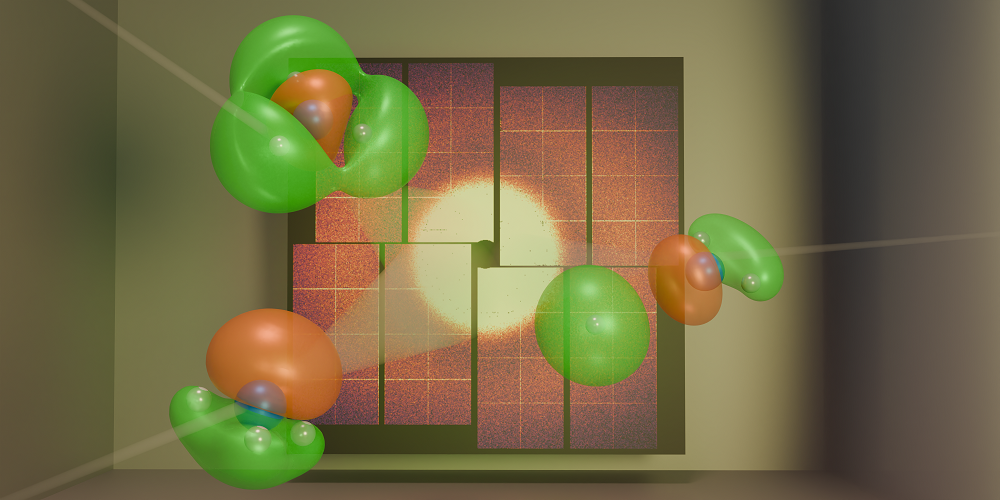
Figure 1: Gabalski and his colleagues fired x-ray pulses at ammonia molecules undergoing a chemical reaction [1]. By analyzing the positions of scattered photons that hit a detector, the researchers were able to track the movement of key electrons during the reaction.
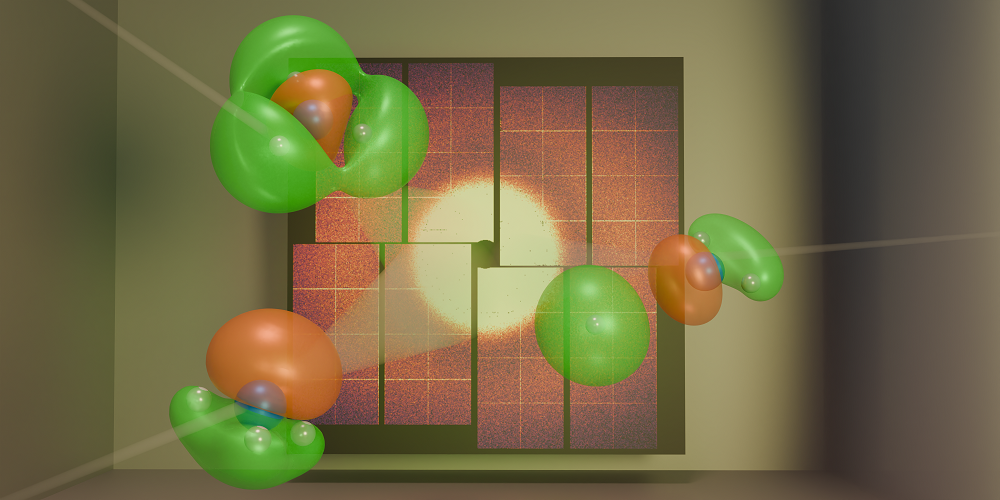
I. Gabalski/Stanford University and SLAC National Accelerator Laboratory
Figure 1: Gabalski and his colleagues fired x-ray pulses at ammonia molecules undergoing a chemical reaction [1]. By analyzing the positions of scattered photons that hit a detector, the researchers were able to track the movement of key electrons during the reaction.×
A chemical reaction occurs when chemical bonds break and new ones form. These bonds hold atoms together within molecules and are governed by the atoms’ outermost electrons. The motion of these so-called valence electrons dictates how a reaction starts and determines its final products. For decades, chemists have envisioned the possibility of watching such electron movement in real time, capturing a movie of valence electrons as bonds break and form. Now Ian Gabalski at Stanford University and his colleagues have brought this dream closer to reality [1]. They have observed valence-electron motion occurring within a few hundred femtoseconds—where one femtosecond is a millionth of a billionth of a second. This feat was accomplished using ultrashort, high-energy x-ray pulses produced at SLAC National Accelerator Laboratory in California. The team’s findings provide an intuitive view of how electron dynamics influence chemical reactions.
Directly observing electron motion during chemical reactions presents two main challenges. First, it requires an imaging technique that can map the spatial distribution of electrons, known as the electron density. This distribution spans only a few tenths of a nanometer, demanding extremely high spatial resolution. Second, the task needs ultrahigh temporal resolution, because electron movement occurs on a timescale of femtoseconds or even attoseconds—thousandths of a femtosecond. Capturing such rapid motion requires the sample to be subjected to light pulses that are short enough to effectively freeze electron dynamics in time, similarly to using a high-speed camera to capture the fluttering wings of a hummingbird.
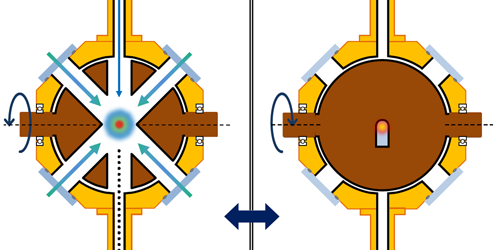
Over the past two decades, an approach has emerged that can meet those challenges: ultrafast x-ray scattering. This technique usually involves a large-scale light source known as an x-ray free-electron laser. This laser produces intense, ultrashort pulses of high-energy x rays that are ideal for capturing the fleeting rearrangement of electrons during a chemical reaction. A typical experiment uses a so-called pump–probe scheme. First, an optical laser pulse excites a molecule, promoting it to a higher-energy electronic state and initiating a chemical reaction. Then, after a controlled time delay, an x-ray pulse probes the molecule. These x-ray photons interact with the electrons inside the molecule and scatter at angles that depend on the electron density. Finally, a large-area 2D detector captures these scattered photons, precisely recording their positions so that a snapshot of the electron density can be reconstructed.
However, the approach has not been able to track valence-electron motion throughout a reaction. In 2015, a proof-of-principle experiment provided a molecular movie of atomic motions, showing how a certain molecule opens its ring structure within 100 femtoseconds (fs) after photoexcitation [2]. Then, a 2020 study advanced that demonstration by capturing the change in valence-electron density immediately after photon absorption, which is considered the first step in such a photochemical reaction [3]. But observing the subsequent evolution of valence-electron motion remained out of reach for ultrafast x-ray scattering. The reason is that most electrons in a molecule are core electrons, which are tightly bound to atomic nuclei and are not directly involved in chemical reactions. The contribution of core electrons to a measured x-ray scattering pattern can mask the subtle changes in the pattern caused by valence-electron motion—especially once significant nuclear motion leads to changes in molecular geometry.
In their work, Gabalski and his colleagues performed an ultrafast x-ray scattering experiment on ammonia molecules (Fig. 1). The researchers used an ultraviolet laser pulse to excite an electron in such a molecule, causing the molecule to unbend from its usual pyramidal structure into a flat, planar geometry. This rearrangement enabled one of the molecule’s three hydrogen atoms to detach, carrying its only electron—a valence electron—with it. Given that a hydrogen atom lacks core electrons, the team was able to track the change in valence-electron density throughout the reaction, even in the presence of significant nuclear motion. This tracking was achieved using high-energy x-ray pulses with a duration of about 30 fs produced by the Linac Coherent Light Source (LCLS), an x-ray free-electron laser at SLAC.
The researchers observed that the hydrogen atom could detach from the molecule through two separate pathways. In each pathway, the valence-electron density had a distinct shape, and signatures of these different shapes persisted for more than 200 fs. These experimental results agreed with the team’s theoretical predictions based on simulations of the quantum dynamics. The findings show that we can now experimentally visualize differences in valence-electron density that directly influence a reaction’s outcome.
A closely related technique, ultrafast electron diffraction, can also be used to create molecular movies. That approach uses high-energy electrons instead of x rays as the probe and has recently been applied to ammonia [4, 5]. Its main limitation is its relatively poor temporal resolution: typically, greater than 50 fs. By comparison, ultrafast x-ray scattering is now advancing toward a resolution below 10 fs.
The next challenge for scientists in this field is to push for attosecond temporal resolution in order to image charge migration and other correlated electron motion. That goal seems attainable given the ongoing development of attosecond x-ray sources at facilities worldwide, such as the LCLS-II at SLAC [6] and the European X-Ray Free-Electron Laser facility located in Germany [7]. Another challenge is to extend these techniques to larger molecules, whose many core electrons can obscure valence-electron signals. A potential way forward is to develop energy-resolved detectors that can distinguish x rays scattered by valence electrons from those scattered by core electrons. Such advances could bring us closer to making detailed molecular movies that capture both electron and nuclear motion as chemical reactions unfold. Ultimately, we might be able to control reaction outcomes by manipulating valence electrons.
References
- I. Gabalski et al., “Imaging valence electron rearrangement in a chemical reaction using hard x-ray scattering,” Phys. Rev. Lett. 135, 083001 (2025).
- M. P. Minitti et al., “Imaging molecular motion: femtosecond x-ray scattering of an electrocyclic chemical reaction,” Phys. Rev. Lett. 114, 255501 (2015).
- H. Yong et al., “Observation of the molecular response to light upon photoexcitation,” Nat. Commun. 11, 2157 (2020).
- E. G. Champenois et al., “Femtosecond electronic and hydrogen structural dynamics in ammonia imaged with ultrafast electron diffraction,” Phys. Rev. Lett. 131, 143001 (2023).
- T. Wang et al., “Probing valence electron and hydrogen dynamics using charge-pair imaging with ultrafast electron diffraction,” arXiv:2506.21047.
- R. Robles et al., “Hard x-ray attosecond pulses at the LCLS,” Proc. SPIE No. 13536, 1353606 (2025).
- J. Yan et al., “Terawatt-attosecond hard x-ray free-electron laser at high repetition rate,” Nat. Photonics 18, 1293 (2024).
About the Author
Haiwang Yong is an assistant professor at the University of California, San Diego. He earned his PhD from Brown University, Rhode Island, in 2021 and was a postdoctoral scholar at the University of California, Irvine (2021–2023). He is a finalist for the LCLS Young Investigator Award at SLAC National Accelerator Laboratory (2021) and a recipient of the Royal Society of Chemistry Faraday Division Horizon Prize (2021). He has served on the Young Editorial Board of Ultrafast Science since 2022. His current research focuses on tracking quantum motions of electrons and nuclei in molecules using ultrafast diffraction and spectroscopic techniques.
Subject AreasRelated Articles
 Atomic and Molecular PhysicsCat Video Made with AtomsAugust 8, 2025
Atomic and Molecular PhysicsCat Video Made with AtomsAugust 8, 2025
To demonstrate a new system for rapidly rearranging thousands of atoms, researchers produced an animation featuring Schrödinger’s famous feline. Read More »