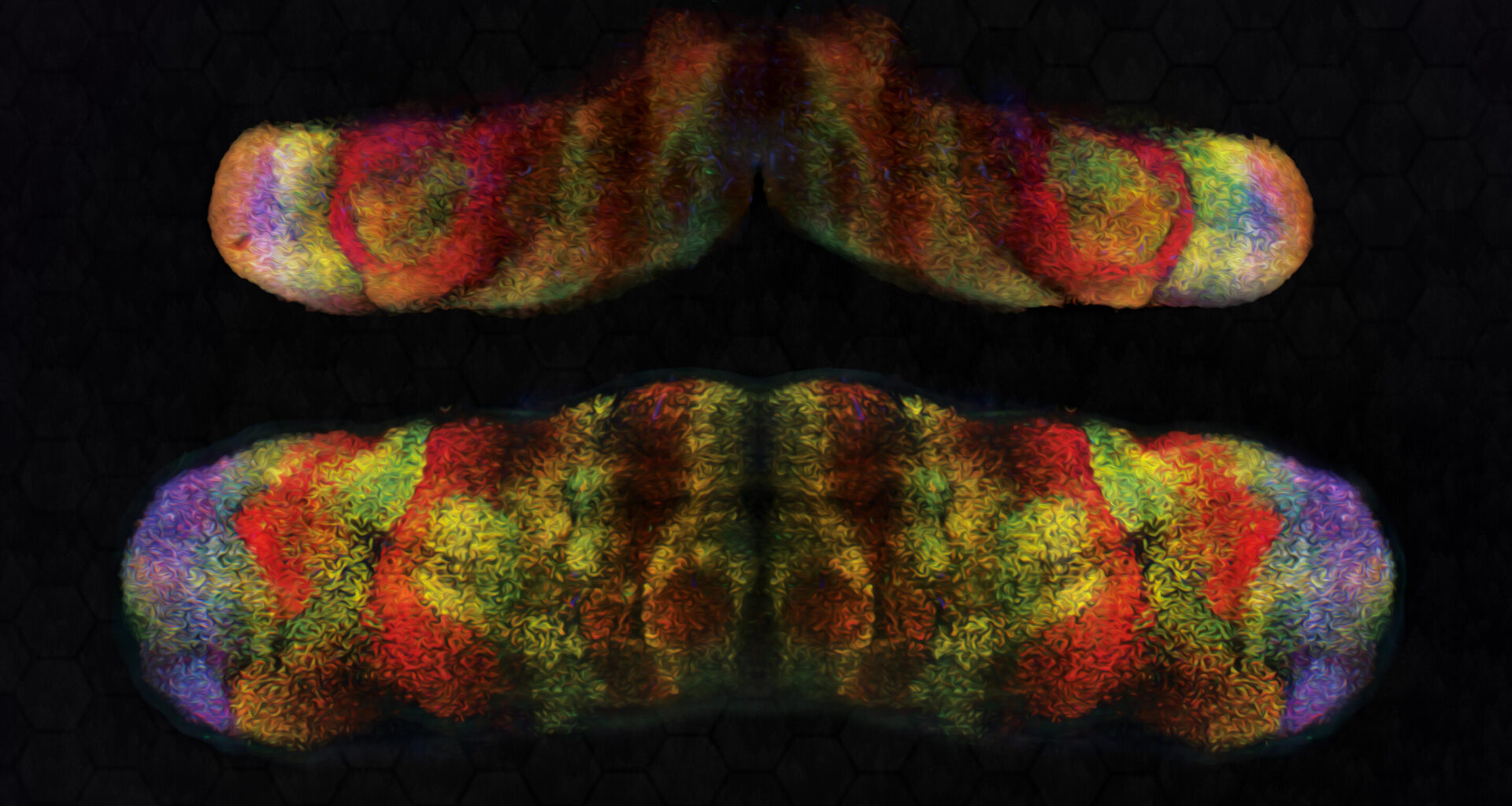
3D light-sheet images of Foxp1 (blue) Foxp2 (red) and Foxp4 (green) in mouse embryonic cerebellum different stages of development (stage in image name; E: embryonic day). Credit: Knouri-Farah et al.
The cerebellum, a brain region located at the back of the head that has long been known to support the coordination of muscle movements, has recently also been implicated in more sophisticated mental functions. Purkinje cells are the only neurons located in the cerebellum that integrate information in the cerebellar cortex and send it to other parts of the nervous system.
Purkinje cells are large and highly branched nerve cells that can have different functions. While many past studies have explored the roles of these cells, the neural and genetic processes shaping their diversity have not yet been fully elucidated.
Researchers at the University of Connecticut School of Medicine recently carried out a study aimed at exploring the possible role of the FOXP genes, a family of genes known to contribute to switching other genes “on and off,” in shaping Purkinje cell populations and the formation of circuits in the cerebellum. Their findings, published in Nature Neuroscience, hint at the existence of at least 11 different Purkinje cell subtypes, suggesting that the FOXP1 and FOXP2 genes contribute to their diversification.
The FOXP family includes four genes, which are known as FOXP1, FOXP2, FOXP3 and FOXP4. Mutations in the FOXP1 and FOXP2 genes have previously been linked to neurodevelopmental and language-related disorders.
“FOXP2 is essential for vocalization across species, supporting song learning in birds, echolocation in bats, pup calling in mice, and proper speech in humans,” James Y.H. Li, senior author of the paper, told Medical Xpress.
“Mutations in FOXP1 cause a syndrome characterized by delayed development, language impairment, intellectual disability, and autism-like traits in humans. Despite their clinical significance, the roles of FOXP genes in brain development have remained poorly understood. We set out to address this gap.”
In their earlier works, Li and his colleagues observed that Foxp1 and Foxp2 were expressed at different levels among the cerebellar Purkinje cells. The main objective of their new study was to determine how the selective deletion of these two genes from the cerebellum would influence the development and mental functions of mice.
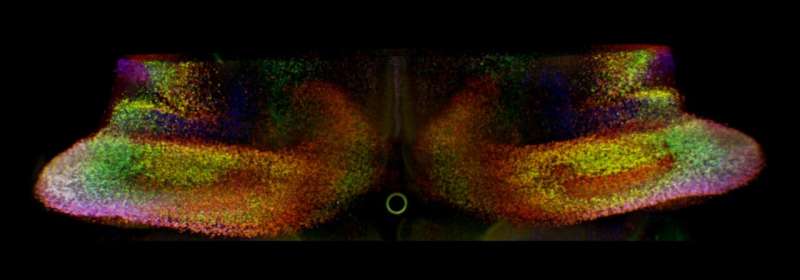
Workflow of scANKRS. Credit: Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02042-w
“Using single-cell RNA sequencing (scRNA-seq), we profiled gene expression in individual cells from embryonic mouse cerebella at key developmental stages,” explained Li. “This revealed multiple molecularly distinct Purkinje cell subtypes. Because scRNA-seq lacks spatial information, we developed scANKRS (single-cell Anchoring Network of Key Regulators to Space), a method that maps scRNA-seq–identified cell groups back into their native tissue context.”
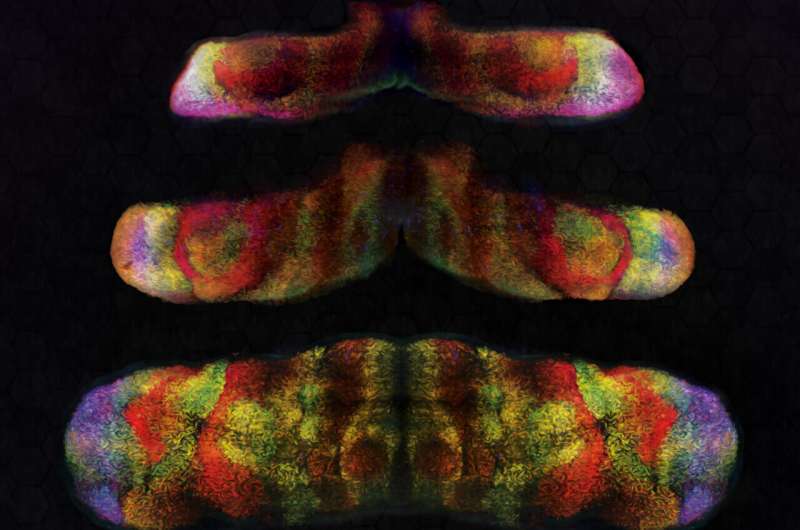
To study the expression of FOXP genes in mice, Li and his colleagues combined spatial mapping of scRNA-seq with another experimental technique known as 3D light-sheet fluorescence imaging. The combination of these two techniques allowed them to directly map the expression patterns of Foxp1, Foxp2, and Foxp4 genes onto emerging Purkinje cell subtypes.
Interestingly, the researchers found that when they deleted Foxp1 or Foxp2 from the mice’s cerebellum, the animals’ ability to produce vocalizations was impaired. This hints at the possible role of the cerebellum in speech and vocalization.
“We also found that Purkinje cells are not all the same, but contain several subtypes with different molecular features, each of which occupies discrete areas in the cerebellar cortex,” said Li. “Since they are the main cells sending signals out of the cerebellar cortex, this diversity lays the foundation for functional specialization of different cortical regions in the cerebellum.”
The experiments carried out by Li and his colleagues also shed light on the processes that could underpin the formation of cerebellar hemispheres. These are bilateral structures that are particularly large in primates and have been linked to advanced abilities, such as sophisticated motor and mental skills.
3D light-sheet images of Foxp1 (blue) Foxp2 (red) and Foxp4 (green) in mouse embryonic cerebellum different stages of development (stage in image name; E: embryonic day). Credit: Knouri-Farah et al.
The researchers found that the deletion of Foxp1 and Foxp2 from the mouse cerebellum prevented the formation of the two hemispheres. Notably, this is the first direct genetic evidence tying hemisphere formation to specific genetic and molecular processes.
“Moreover, we observed that Foxp1-positive Purkinje cells, the subtype most affected by the knockouts, are abundant in the human fetal cerebellum but rare in birds,” said Li. “Their presence may have driven the evolutionary expansion of mammalian cerebellar hemispheres, enabling the development of higher cognitive functions.”
The recent work by Li and his colleagues gathered strong evidence suggesting that FOXP genes contribute to the diversification of Purkinje cells into different subtypes and to the establishment of connections to other regions within and outside of the cerebellum. If validated in future studies involving primates and humans, the team’s findings could offer a new explanation for the link between some neurodevelopmental disorders, including autism spectrum disorder (ASD) and FOXP insufficiency.
“Our next steps will be to define the molecular mechanisms of FOXP transcription factors—their binding partners, target genes, and regulatory networks,” added Li. “The discovery of Purkinje cell diversity now allows us to test how perturbing specific subtypes, particularly Foxp1-positive Purkinje cells, influences cerebellar hemisphere expansion.”
Written for you by our author Ingrid Fadelli, edited by Gaby Clark, and fact-checked and reviewed by Robert Egan—this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive.
If this reporting matters to you,
please consider a donation (especially monthly).
You’ll get an ad-free account as a thank-you.
More information:
Nagham Khouri-Farah et al, FOXP genes regulate Purkinje cell diversity and cerebellar morphogenesis, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02042-w. www.nature.com/articles/s41593-025-02042-w
© 2025 Science X Network
Citation:
Genetic deletion in cerebellum impedes hemisphere formation, study finds (2025, September 12)
retrieved 13 September 2025
from https://medicalxpress.com/news/2025-09-genetic-deletion-cerebellum-impedes-hemisphere.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.