Stark, Z. et al. Integrating genomics into healthcare: a global responsibility. Am. J. Hum. Genet 104, 13–20 (2019).
Sheikh Hassani, M. et al. Virtual gene panels have a superior diagnostic yield for inherited rare diseases relative to static panels. Clin. Chem. 71, 169–184 (2025).
Wojcik, M. H. et al. Genome sequencing for diagnosing rare diseases. N. Engl. J. Med. 390, 1985–1997 (2024).
Dai, P. et al. Recommendations for next generation sequencing data reanalysis of unsolved cases with suspected Mendelian disorders: a systematic review and meta-analysis. Genet. Med. 24, 1618–1629 (2022).
Martyn, M. et al. Offering complex genomic screening in acute pediatric settings: Family decision-making and outcomes. Genet. Med. 27, 101327 (2024).
Leong, I. U. S. et al. Large-scale pharmacogenomics analysis of patients with cancer within the 100,000 Genomes Project combining whole-genome sequencing and medical records to inform clinical practice. J. Clin. Oncol. 43, 682–693 (2025).
Chen, Y. et al. De novo variants in the RNU4-2 snRNA cause a frequent neurodevelopmental syndrome. Nature 632, 832–840 (2024).
Clark, M. M. et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 3, 16 (2018).
Kohler, J. N., Turbitt, E. & Biesecker, B. B. Personal utility in genomic testing: a systematic literature review. Eur. J. Hum. Genet 25, 662–668 (2017).
Best, S. et al. Clinical genomic testing: what matters to key stakeholders?. Eur. J. Hum. Genet. 28, 866–873 (2020).
Pollard, S. et al. Toward the diagnosis of rare childhood genetic diseases: what do parents value most?. Eur. J. Hum. Genet. 29, 1491–1501 (2021).
Smith, H. S. et al. Conceptualization of utility in translational clinical genomics research. Am. J. Hum. Genet. 108, 2027–2036 (2021).
Smith, H. S. et al. Key drivers of family-level utility of pediatric genomic sequencing: a qualitative analysis to support preference research. Eur. J. Hum. Genet. 31, 445–452 (2023).
ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet. Med. 17, 505–507 (2015).
Norris, S., Belcher, A., Howard, K. & Ward, R. L. Evaluating genetic and genomic tests for heritable conditions in Australia: lessons learnt from health technology assessments. J. Community Genet. 13, 503–522 (2022).
Ellard, S. et al. Rare disease genomic testing in the UK and Ireland: promoting timely and equitable access. J. Med. Genet. 61, 1103–1112 (2024).
Expert Meeting on Accelerating Access to Human Genomics for Public Health Participants. Accelerating access to human genomics for public health: perspectives from the Western Pacific region. Lancet Reg. Health West Pac. 53, 101265 (2024).
Wu, Y. et al. Parental health spillover effects of paediatric rare genetic conditions. Qual. Life Res. 29, 2445–2454 (2020).
National Institute for Health and Care Excellence. Modular update to NICE manuals: health inequalities. https://www.nice.org.uk/process/pmg36/documents/supporting-documentation-3 (2025).
Hayeems, R. Z. et al. Clinical utility of genomic sequencing: a measurement toolkit. NPJ Genom. Med. 5, 56 (2020).
Mallett, A., Stark, Z., Fehlberg, Z., Best, S. & Goranitis, I. Determining the utility of diagnostic genomics: a conceptual framework. Hum. Genomics 17, 75 (2023).
Chung, C. C. Y., Chu, A. T .W. & Chung, B. H. Y. A roadmap for genome projects to foster psychosocial and economic evidence to further policy and practice. Commun. Med. 5, 198 (2025).
Hartley, T. et al. Evaluation of the diagnostic accuracy of exome sequencing and its impact on diagnostic thinking for patients with rare disease in a publicly funded health care system: a prospective cohort study. Genet. Med. 26, 101012 (2024).
Australian Genomics Health Alliance Acute Care Flagship; Lunke, S. et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA 323, 2503–2511 (2020).
Lunke, S. et al. Integrated multi-omics for rapid rare disease diagnosis on a national scale. Nat. Med. 29, 1681–1691 (2023).
Dimmock, D. et al. Project Baby Bear: rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am. J. Hum. Genet. 108, 1231–1238 (2021).
Grant, P. E., Pampaka, M., Payne, K., Clarke, A. & McAllister, M. Developing a short-form of the Genetic Counselling Outcome Scale: The Genomics Outcome Scale. Eur. J. Med Genet. 62, 324–334 (2019).
Stark, Z. et al. Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet. Med. 21, 173–180 (2019).
Khoury, M. J. et al. Health equity in the implementation of genomics and precision medicine: a public health imperative. Genet. Med. 24, 1630–1639 (2022).
McAllister, M., Wood, A. M., Dunn, G., Shiloh, S. & Todd, C. The Genetic Counseling Outcome Scale: a new patient-reported outcome measure for clinical genetics services. Clin. Genet. 79, 413–424 (2011).
Smith, H. S. et al. Clinical application of genome and exome sequencing as a diagnostic tool for pediatric patients: a scoping review of the literature. Genet. Med. 21, 3–16 (2019).
Pan, T., Wu, Y., Buchanan, J. & Goranitis, I. QALYs and rare diseases: exploring the responsiveness of SF-6D, EQ-5D-5L and AQoL-8D following genomic testing for childhood and adult-onset rare genetic conditions in Australia. Health Qual. Life Outcomes 21, 132 (2023).
Hayeems, R. Z. et al. The development of the Clinician-reported Genetic testing Utility InDEx (C-GUIDE): a novel strategy for measuring the clinical utility of genetic testing. Genet. Med. 22, 95–101 (2020).
Hayeems, R. Z. et al. The Clinician-reported Genetic testing Utility InDEx (C-GUIDE): preliminary evidence of validity and reliability. Genet. Med. 24, 430–438 (2022).
Hayeems, R. Z. et al. Assessing the performance of the Clinician-reported Genetic Testing Utility InDEx (C-GUIDE): further evidence of inter-rater reliability. Clin. Ther. 45, 729–735 (2023).
Hayeems, R. Z. et al. Applying the Clinician-reported Genetic testing Utility InDEx (C-GUIDE) to genome sequencing: further evidence of validity. Eur. J. Hum. Genet. 30, 1423–1431 (2022).
Hayeems, R. Z. et al. The Clinician-reported Genetic Testing Utility InDEx (C-GUIDE) for Prenatal Care: initial evidence of content and construct validity. Genet. Med. 27, 101306 (2025).
Dolman, L. I. et al. The Clinician-reported Genetic Testing Utility InDEx for Neonatal Intensive Care (C-GUIDE NICU): quantifying genome-wide sequencing utility in the NICU. Genet. Med. 27, 101503 (2025).
Smith, H. S. et al. Perceived utility of genomic sequencing: qualitative analysis and synthesis of a conceptual model to inform patient-centered instrument development. Patient 15, 317–328 (2022).
Smith, H. S. et al. Measuring perceived utility of genomic sequencing: Development and validation of the GENEtic Utility (GENE-U) scale for pediatric diagnostic testing. Genet. Med. 26, 101146 (2024).
Smith, H. S. et al. Measuring perceived utility of genomic sequencing: Development and validation of the GENEtic Utility (GENE-U) scale for adult screening. Genet. Med. 26, 101240 (2024).
Turbitt, E. et al. The PrU: development and validation of a measure to assess personal utility of genomic results. Genet. Med. 25, 100356 (2022).
Turbitt, E. et al. The Parent PrU: a measure to assess personal utility of pediatric genomic results. Genet. Med. 26, 100994 (2024).
Fehlberg, Z., Goranitis, I., Mallett, A. J., Stark, Z. & Best, S. Determining priority indicators of utility for genomic testing in rare disease: a Delphi study. Genet. Med. 26, 101116 (2024).
Goranitis, I. et al. A standardized measurement and valuation scale of genomic utility for policy decisions: The GUV Scale. Value Health 28, 184–190 (2025).
Phillips, K. A. et al. Methodological issues in assessing the economic value of next-generation sequencing tests: many challenges and not enough solutions. Value Health 21, 1033–1042 (2018).
Grosse, S. D. & Khoury, M. J. What is the clinical utility of genetic testing?. Genet. Med. 8, 448–450 (2006).
Grosse, S. D., Wordsworth, S. & Payne, K. Economic methods for valuing the outcomes of genetic testing: beyond cost-effectiveness analysis. Genet. Med. 10, 648–654 (2008).
Regier, D. A., Friedman, J. M., Makela, N., Ryan, M. & Marra, C. A. Valuing the benefit of diagnostic testing for genetic causes of idiopathic developmental disability: willingness to pay from families of affected children. Clin. Genet. 75, 514–521 (2009).
Regier, D. A., Ryan, M., Phimister, E. & Marra, C. A. Bayesian and classical estimation of mixed logit: an application to genetic testing. J. Health Econ. 28, 598–610 (2009).
Ho, M. et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies. Value Health 19, 746–750 (2016).
Bridges, J. F. P. et al. A roadmap for increasing the usefulness and impact of patient-preference studies in decision making in health: a good practices report of an ISPOR task force. Value Health 26, 153–162 (2023).
Smith, H. S. et al. Approaches to incorporation of preferences into health economic models of genomic medicine: a critical interpretive synthesis and conceptual framework. Appl. Health Econ. Health Policy 23, 337–358 (2025).
Strauss, K. A. et al. Genomic diagnostics within a medically underserved population: efficacy and implications. Genet. Med. 20, 31–41 (2018).
Marshall, D. A. et al. The value of diagnostic testing for parents of children with rare genetic diseases. Genet. Med. 21, 2798–2806 (2019).
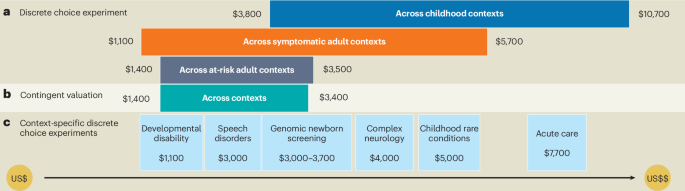
Meng, Y., Clarke, P. M. & Goranitis, I. The value of genomic testing: a contingent valuation across six child- and adult-onset genetic conditions. Pharmacoeconomics 40, 215–223 (2022).
Goranitis, I., Best, S., Christodoulou, J., Stark, Z. & Boughtwood, T. The personal utility and uptake of genomic sequencing in pediatric and adult conditions: eliciting societal preferences with three discrete choice experiments. Genet. Med. 22, 1311–1319 (2020).
Goranitis, I., Best, S., Stark, Z., Boughtwood, T. & Christodoulou, J. The value of genomic sequencing in complex pediatric neurological disorders: a discrete choice experiment. Genet. Med. 23, 155–162 (2021).
Goranitis, I., Best, S., Christodoulou, J., Boughtwood, T. & Stark, Z. Preferences and values for rapid genomic testing in critically ill infants and children: a discrete choice experiment. Eur. J. Hum. Genet. 29, 1645–1653 (2021).
Meng, Y. et al. The value of genomic testing in severe childhood speech disorders. Eur. J. Hum. Genet. 32, 440–447 (2024).
Regier, D. A., Weymann, D., Buchanan, J., Marshall, D. A. & Wordsworth, S. Valuation of health and nonhealth outcomes from next-generation sequencing: approaches, challenges, and solutions. Value Health 21, 1043–1047 (2018).
Peters, R. et al. Public preferences for the value and implementation of genomic newborn screening: Insights from two discrete choice experiments in Australia. Am. J. Hum. Genet. 112, 1515–1527 (2025).
Regier, D. A., Friedman, J. M. & Marra, C. A. Value for money? Array genomic hybridization for diagnostic testing for genetic causes of intellectual disability. Am. J. Hum. Genet. 86, 765–772 (2010).
Goranitis, I. et al. Is faster better? An economic evaluation of rapid and ultra-rapid genomic testing in critically ill infants and children. Genet. Med. 24, 1037–1044 (2022).
Wu, Y. et al. Genomic sequencing for the diagnosis of childhood mitochondrial disorders: a health economic evaluation. Eur. J. Hum. Genet. 30, 577–586 (2022).
Downie, L. et al. Exome sequencing for isolated congenital hearing loss: a cost-effectiveness analysis. Laryngoscope 131, E2371–E2377 (2021).
Jayasinghe, K. et al. Cost-effectiveness of targeted exome analysis as a diagnostic test in glomerular diseases. Kidney Int. Rep. 6, 2850–2861 (2021).
O’Rourke, B., Oortwijn, W. Schuller, T. & International Joint Task Group The new definition of health technology assessment:a milestone in international collaboration. Int. J. Technol. Assess. Health Care 36, 187–190 (2020).
Group, N. I. S. et al. Effect of whole-genome sequencing on the clinical management of acutely ill infants with suspected genetic disease: a randomized clinical trial. JAMA Pediatr. 175, 1218–1226 (2021).
Kingsmore, S. F. et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in ill infants. Am. J. Hum. Genet. 105, 719–733 (2019).
Dimmock, D. P. et al. An RCT of rapid genomic sequencing among seriously ill infants results in high clinical utility, changes in management, and low perceived harm. Am. J. Hum. Genet. 107, 942–952 (2020).
Petrikin, J. E. et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom. Med. 3, 6 (2018).
Le Tourneau, C. et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Ceyhan-Birsoy, O. et al. Interpretation of genomic sequencing results in healthy and ill newborns: results from the BabySeq Project. Am. J. Hum. Genet. 104, 76–93 (2019).
Gibbs, S. N. et al. Comprehensive review on the clinical impact of next-generation sequencing tests for the management of advanced cancer. JCO Precis. Oncol. 7, e2200715 (2023).
Stark, Z. et al. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet. Med. 19, 867–874 (2017).
Tan, T. Y. et al. A head-to-head evaluation of the diagnostic efficacy and costs of trio versus singleton exome sequencing analysis. Eur. J. Hum. Genet. 27, 1791–1799 (2019).
Tuxen, I. V. et al. Copenhagen Prospective Personalized Oncology (CoPPO)-clinical utility of using molecular profiling to select patients to phase I trials. Clin. Cancer Res. 25, 1239–1247 (2019).
Von Hoff, D. D. et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 28, 4877–4883 (2010).
Weymann, D. et al. Clinical and cost outcomes following genomics-informed treatment for advanced cancers. Cancer Med. 10, 5131–5140 (2021).
Vissers, L. et al. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet. Med. 19, 1055–1063 (2017).
Yeung, A. et al. A cost-effectiveness analysis of genomic sequencing in a prospective versus historical cohort of complex pediatric patients. Genet. Med. 22, 1986–1993 (2020).
Weymann, D. et al. Early-stage economic analysis of research-based comprehensive genomic sequencing for advanced cancer care. J. Community Genet. 13, 523–538 (2022).
Hernando-Calvo, A. et al. Impact on costs and outcomes of multi-gene panel testing for advanced solid malignancies: a cost-consequence analysis using linked administrative data. EClinicalMedicine 69, 102443 (2024).
Krebs, E. et al. Real-world cost-effectiveness of multi-gene panel sequencing to inform therapeutic decisions for advanced non-small cell lung cancer: a population-based study. Lancet Reg. Health Am. 40, 100936 (2024).
Chung, C. C. Y. et al. Meta-analysis of the diagnostic and clinical utility of exome and genome sequencing in pediatric and adult patients with rare diseases across diverse populations. Genet. Med. 25, 100896 (2023).
Shickh, S., Mighton, C., Uleryk, E., Pechlivanoglou, P. & Bombard, Y. The clinical utility of exome and genome sequencing across clinical indications: a systematic review. Hum. Genet. 140, 1403–1416 (2021).
Kobayashi, E. S. et al. Long term follow up of children who received rapid genomic sequencing. Genet. Med. 27, 101403 (2025).
Payne, K., Gavan, S. P., Wright, S. J. & Thompson, A. J. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat. Rev. Genet. 19, 235–246 (2018).
Schwarze, K., Buchanan, J., Taylor, J. C. & Wordsworth, S. Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet. Med. 20, 1122–1130 (2018).
Grosse, S. D. & Gudgeon, J. M. Cost or price of sequencing? Implications for economic evaluations in genomic medicine. Genet. Med. 23, 1833–1835 (2021).
Santos Gonzalez, F. et al. Microcosting diagnostic genomic sequencing: a systematic review. Genet. Med. 25, 100829 (2023).
Fahr, P., Buchanan, J. & Wordsworth, S. A review of health economic studies comparing traditional and massively parallel sequencing diagnostic pathways for suspected genetic disorders. Pharmacoeconomics 38, 143–158 (2020).
Santos Gonzalez, F. et al. Microcosting genomics: challenges and opportunities. Genet. Med. 27, 101310 (2025).
Bourke, M. et al. The cost-effectiveness of genomic medicine in cancer control: a systematic literature review. Appl. Health Econ. Health Policy 23, 359–393 (2025).
Regier, D. A. et al. A perspective on life-cycle health technology assessment and real-world evidence for precision oncology in Canada. NPJ Precis. Oncol. 6, 76 (2022).
Schmidtke, J. & Cassiman, J. J. The EuroGentest clinical utility gene cards. Eur. J. Hum. Genet. 18, 1068 (2010).
Burke, W. et al. Genetic test evaluation: information needs of clinicians, policy makers, and the public. Am. J. Epidemiol. 156, 311–318 (2002).
Schilling, C. et al. Utilisation of subsidised genetic and genomic testing in a publicly funded healthcare system 2014-2023. Eur. J. Hum. Genet. 33, 1044–1050 (2025).
Mordaunt, D. A., Dalziel, K., Goranitis, I. & Stark, Z. Uptake of funded genomic testing for syndromic and non-syndromic intellectual disability in Australia. Eur. J. Hum. Genet 31, 977–979 (2023).
Douglas, M. P., Parker, S. L., Trosman, J. R., Slavotinek, A. M. & Phillips, K. A. Private payer coverage policies for exome sequencing (ES) in pediatric patients: trends over time and analysis of evidence cited. Genet. Med. 21, 152–160 (2019).
Trosman, J. R. et al. Perspectives of US private payers on insurance coverage for pediatric and prenatal exome sequencing: results of a study from the Program in Prenatal and Pediatric Genomic Sequencing (P3EGS). Genet. Med. 22, 283–291 (2020).
Smith, H. S. et al. Outcomes of prior authorization requests for genetic testing in outpatient pediatric genetics clinics. Genet. Med. 23, 950–955 (2021).
Zion, T. N. et al. Insurance denials and diagnostic rates in a pediatric genomic research cohort. Genet. Med. 25, 100020 (2023).
Lu, C. Y. et al. Insurance coverage policies for pharmacogenomic and multi-gene testing for cancer. J. Pers. Med. 8, 19 (2018).
Beinfeld, M. T., Rucker, J. A., Jenkins, N. B., de Breed, L. A. & Chambers, J. D. Variation in Medicaid and commercial coverage of cell and gene therapies. Health Policy Open 5, 100103 (2023).
Phillips, K. A. et al. US private payers’ perspectives on insurance coverage for genome sequencing versus exome sequencing: a study by the Clinical Sequencing Evidence-Generating Research Consortium (CSER). Genet. Med. 24, 238–244 (2022).
Smith, H. S., Sherman, M. & Cardeiro, D. Conversations with the editors: stewardship in genomic medicine-insights from health care payers at the forefront of clinical innovation and partnerships. Clin. Ther. 45, 690–694 (2023).
Wiedower, J. et al. Payer perspectives on genomic testing in the United States: a systematic literature review. Genet. Med. 27, 101329 (2025).
Braithwaite, J., Churruca, K., Long, J. C., Ellis, L. A. & Herkes, J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med. 16, 63 (2018).
Zebrowski, A. M. et al. Qualitative study of system-level factors related to genomic implementation. Genet. Med. 21, 1534–1540 (2019).
Best, S., Long, J. C., Gaff, C., Braithwaite, J. & Taylor, N. Investigating the adoption of clinical genomics in Australia. An Implementation Science case study. Genes 12, 317 (2021).
Gaff, C. L. et al. Preparing for genomic medicine: a real world demonstration of health system change. NPJ Genom. Med. 2, 16 (2017).
Best, S., Long, J. C., Gaff, C., Braithwaite, J. & Taylor, N. Organizational perspectives on implementing complex health interventions: clinical genomics in Australia. J. Health Organ. Manag. 35, 825–845 (2021).
Stark, Z. et al. Australian genomics: outcomes of a 5-year national program to accelerate the integration of genomics in healthcare. Am. J. Hum. Genet. 110, 419–426 (2023).
Braithwaite, J., Glasziou, P. & Westbrook, J. The three numbers you need to know about healthcare: the 60-30-10 challenge. BMC Med. 18, 102 (2020).
Khorshidi, H. A., Marshall, D., Goranitis, I., Schroeder, B. & IJzerman, M. System dynamics simulation for evaluating implementation strategies of genomic sequencing: tutorial and conceptual model. Expert Rev. Pharmacoecon. Outcomes Res. 24, 37–47 (2024).
Investigators, G. P. P. et al. 100,000 genomes pilot on rare-disease diagnosis in health care – preliminary report. N. Engl. J. Med. 385, 1868–1880 (2021).
Sosinsky, A. et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 30, 279–289 (2024).
Howley, C. et al. The expanding global genomics landscape: converging priorities from national genomics programs. Am. J. Hum. Genet. 112, 751–763 (2025).
Stark, Z. et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet. Med. 18, 1090–1096 (2016).
Lionel, A. C. et al. Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test. Genet. Med. 20, 435–443 (2018).
Regier, D. A. et al. Real-world diagnostic outcomes and cost-effectiveness of genome-wide sequencing for developmental and seizure disorders: evidence from Canada. Genet. Med. 26, 101069 (2024).
Dragojlovic, N. et al. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet. Med. 22, 1437–1449 (2020).
Jenkins, B. D. et al. The 2019 US medical genetics workforce: a focus on clinical genetics. Genet. Med. 23, 1458–1464 (2021).
Jayasinghe, K. et al. Clinical impact of genomic testing in patients with suspected monogenic kidney disease. Genet. Med. 23, 183–191 (2021).
Yanes, T. et al. Evaluation and pilot testing of a multidisciplinary model of care to mainstream genomic testing for paediatric inborn errors of immunity. Eur. J. Hum. Genet 31, 1125–1132 (2023).
Ma, A. et al. Genomic multidisciplinary teams: a model for navigating genetic mainstreaming and precision medicine. J. Paediatr. Child Health 60, 118–124 (2024).
McCorkell, G. et al. A national education program for rapid genomics in pediatric acute care: Building workforce confidence, competence, and capability. Genet. Med. 26, 101224 (2024).
Nisselle, A., Terrill, B., Janinski, M., Metcalfe, S. & Gaff, C. Ensuring best practice in genomics education: a scoping review of genomics education needs assessments and evaluations. Am. J. Hum. Genet. 111, 1508–1523 (2024).
Nisselle, A. et al. Ensuring best practice in genomics education: a theory- and empirically informed evaluation framework. Am. J. Hum. Genet. 111, 1497–1507 (2024).
Nightingale, K. P. et al. Evaluation of the Master’s in Genomic Medicine framework: a national, multiprofessional program to educate health care professionals in NHS England. Genet. Med. 27, 101277 (2025).
Pichini, A. & Bishop, M. A nationally agreed cross-professional competency framework to facilitate genomic testing. Genet. Med. 24, 1743–1752 (2022).
Pichini, A., Tatton-Brown, K., Thomas, E. & Bishop, M. A cross-professional competency framework for communicating genomic results. J. Genet. Couns. 33, 222–231 (2024).
Karthikeyan, A., McKee, S. & McKay, G. J. Integration of genomic medicine to mainstream patient care within the UK National Health Service. Ulster Med. J. 93, 111–118 (2024).
Berkman, J. et al. Mainstreaming cancer genomic testing: a scoping review of the acceptability, efficacy, and impact. Clin. Genet. 107, 123–135 (2025).
Mackley, M. P. et al. Mainstreaming of clinical genetic testing: a conceptual framework. Genet. Med. 27, 101465 (2025).
Mordaunt, D. A. et al. The cost of proband and trio exome and genome analysis in rare disease: a micro-costing study. Genet. Med. 26, 101058 (2024).
Best, S. et al. Teamwork in clinical genomics: a dynamic sociotechnical healthcare setting. J. Eval. Clin. Pract. 27, 1369–1380 (2021).
Best, S. et al. The leadership behaviors needed to implement clinical genomics at scale: a qualitative study. Genet. Med. 22, 1384–1390 (2020).
Friedrich, B. et al. A very big challenge”: a qualitative study to explore the early barriers and enablers to implementing a national genomic medicine service in England. Front. Genet. 14, 1282034 (2023).
Kirk, E. P. et al. Nationwide, couple-based genetic carrier screening. N. Engl. J. Med. 391, 1877–1889 (2024).
Casalino, S. et al. Genome screening, reporting, and genetic counseling for healthy populations. Hum. Genet. 142, 181–192 (2023).
Stark, Z. & Scott, R. H. Genomic newborn screening for rare diseases. Nat. Rev. Genet. 24, 755–766 (2023).
McDermott, J. H., Tsakiroglou, M., Newman, W. G. & Pirmohamed, M. Pharmacogenomics in the UK National Health Service: progress towards implementation. Br. J. Clin. Pharmacol. 91, 2241–2250 (2025).
Lennon, N. J. et al. Selection, optimization and validation of ten chronic disease polygenic risk scores for clinical implementation in diverse US populations. Nat. Med. 30, 480–487 (2024).
Rehm, H. L. et al. ClinGen–the Clinical Genome Resource. N. Engl. J. Med. 372, 2235–2242 (2015).
Rehm, H. L., Harrison, S. M. & Martin, C. L. ClinVar is a critical resource to advance variant interpretation. Oncologist 22, 1562 (2017).
Tudini, E. et al. Shariant platform: enabling evidence sharing across Australian clinical genetic-testing laboratories to support variant interpretation. Am. J. Hum. Genet 109, 1960–1973 (2022).
Stark, Z. et al. Scaling national and international improvement in virtual gene panel curation via a collaborative approach to discordance resolution. Am. J. Hum. Genet. 108, 1551–1557 (2021).
DiStefano, M. T. et al. The Gene Curation Coalition: a global effort to harmonize gene-disease evidence resources. Genet. Med. 24, 1732–1742 (2022).
Stark, Z. et al. A call to action to scale up research and clinical genomic data sharing. Nat. Rev. Genet. 26, 141–147 (2025).
Rehm, H. L. et al. GA4GH: international policies and standards for data sharing across genomic research and healthcare. Cell. Genom. 1, 100029 (2021).
Roberts, M. C., Kennedy, A. E., Chambers, D. A. & Khoury, M. J. The current state of implementation science in genomic medicine: opportunities for improvement. Genet. Med. 19, 858–863 (2017).
Chambers, D. A., Feero, W. G. & Khoury, M. J. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA 315, 1941–1942 (2016).
Moullin, J. C. et al. Ten recommendations for using implementation frameworks in research and practice. Implement. Sci. Commun. 1, 42 (2020).
Brown, H. L., Sherburn, I. A., Gaff, C., Taylor, N. & Best, S. Structured approaches to implementation of clinical genomics: a scoping review. Genet Med 24, 1415–1424 (2022).
Curran, G. M., Bauer, M., Mittman, B., Pyne, J. M. & Stetler, C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care 50, 217–226 (2012).
Taylor, N. et al. Aligning intuition and theory: a novel approach to identifying the determinants of behaviours necessary to support implementation of evidence into practice. Implement. Sci. 18, 29 (2023).
Best, S. et al. Learning from scaling up ultra-rapid genomic testing for critically ill children to a national level. NPJ Genom. Med. 6, 5 (2021).
Cheng, L. et al. Optimising the mainstreaming of renal genomics: Complementing empirical and theoretical strategies for implementation. Eur. J. Hum. Genet. 33, 351–359 (2025).
Best, S. et al. Using a theory informed approach to design, execute, and evaluate implementation strategies to support offering reproductive genetic carrier screening in Australia. BMC Health Serv. Res. 23, 1276 (2023).
Fehlberg, Z. et al. Scaling-up and future sustainability of a national reproductive genetic carrier screening program. NPJ Genom. Med. 8, 18 (2023).
Best, S., Long, J. C., Braithwaite, J. & Taylor, N. Standardizing variation: Scaling up clinical genomics in Australia. Genet. Med. 25, 100109 (2023).
Laskowski, N. M. et al. Variation exists in service delivery: similarities and differences in the provision of a whole genome sequencing service for paediatric rare disease patients in the National Health Service in England. Public Health Genomics 28, 1–18 (2025).
Best, S., Vidic, N., An, K., Collins, F. & White, S. M. A systematic review of geographical inequities for accessing clinical genomic and genetic services for non-cancer related rare disease. Eur. J. Hum. Genet. 30, 645–652 (2022).
Casauria, S. et al. Assessing the unmet needs of genomic testing in Australia: a geospatial exploration. Eur. J. Hum. Genet. 33, 496–503 (2024).
Pichler, F. B. et al. An operationalization framework for lifecycle health technology assessment: a Health Technology Assessment International Global Policy Forum Task Force report. Int. J. Technol. Assess. Health Care 40, e45 (2024).
Makady, A., de Boer, A., Hillege, H., Klungel, O. & Goettsch, W. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health 20, 858–865 (2017).
Sterrantino, A. F. Observational studies: practical tips for avoiding common statistical pitfalls. Lancet Reg. Health Southeast Asia 25, 100415 (2024).
Weymann, D., Krebs, E. & Regier, D. A. Addressing immortal time bias in precision medicine: practical guidance and methods development. Health Serv. Res. 60, e14376 (2025).
Hernan, M. A., Sauer, B. C., Hernandez-Diaz, S., Platt, R. & Shrier, I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J. Clin. Epidemiol. 79, 70–75 (2016).
Pollard, S. et al. Defining a core data set for the economic evaluation of precision oncology. Value Health 25, 1371–1380 (2022).
McPhail, M., McCabe, C., Regier, D. A. & Bubela, T. The importance of and challenges with adopting life-cycle regulation and reimbursement in Canada. Health. Policy 17, 81–90 (2022).
Maggio, L. A., Villalba, K., German, D., Kanter, S. L. & Collard, H. R. Defining the learning health care system: an international health system leadership perspective. Acad. Med. 99, 215–220 (2024).
Meghea, C. I. et al. An NIH investment in health equity – the economic impact of the Flint Center for Health Equity Solutions. BMC Public Health 21, 1774 (2021).
Hock, D. H. et al. Untargeted proteomics enables ultra-rapid variant prioritisation in mitochondrial and other rare diseases. Genome Med. 17, 58 (2025).
Tambuyzer, E. et al. Therapies for rare diseases: therapeutic modalities, progress and challenges ahead. Nat. Rev. Drug Discov. 19, 93–111 (2020).
Chung, C. C. Y., Chu, A. T. W. & Chung, B. H. Y. A roadmap for genome projects to foster psychosocial and economic evidence to further policy and practice. Commun. Med. 5, 198 (2025).