The capsule, built with a zinc-cellulose antenna and an ultra-small RFID chip, emits a radio signal once swallowed, allowing healthcare providers to verify medication intake in real time. Most of the device safely degrades inside the digestive tract, with only the tiny chip passing through the body.
This type of system could be especially valuable for patients who require strict medication adherence, such as those recovering from organ transplants or undergoing long-term treatment for infections like HIV or tuberculosis. According to a study published in Nature Communications, failure to take medication as prescribed is a widespread issue that contributes to over 125,000 preventable deaths and more than $100 billion in avoidable healthcare costs every year in the United States alone.
The project, led by MIT’s Giovanni Traverso, also a gastroenterologist at Brigham and Women’s Hospital, is part of a broader push to develop patient-friendly monitoring tools that don’t rely on wearable devices or invasive procedures. Researchers believe that integrating these capsules into existing treatment plans could help bridge critical gaps in medication adherence, especially in vulnerable or high-risk populations.
A Bioresorbable System Designed for Patient Safety
The capsule’s key feature lies in its degradability. The zinc-based antenna is embedded in a cellulose matrix and paired with a micro-RFID chip that does not require a battery. As detailed in the MIT press release, the outer coating (made from gelatin and a biodegradable metal such as molybdenum or tungsten) forms a Faraday cage that initially blocks signal emission.

Once ingested, the capsule begins to dissolve in the stomach. Within minutes, the shielding layer breaks down, activating the antenna and triggering a signal to an external reader. The signal is detected up to two feet away and can confirm the pill’s ingestion within 10 minutes.
According to the research team, nearly all components of the system degrade within the body over the course of a few days, except the RFID chip, which is safely excreted. Mehmet Girayhan Say, one of the lead authors, emphasized that the materials were selected for their safety profiles and compatibility with medical and environmental standards.
Clinical Applications and Real-Time Monitoring Potential
This technology is not designed for casual over-the-counter use, but rather for clinical settings where medication adherence is critical. One such population is transplant recipients, who must take immunosuppressants on a strict schedule to prevent organ rejection. Another target group includes people undergoing treatment for chronic infectious diseases.
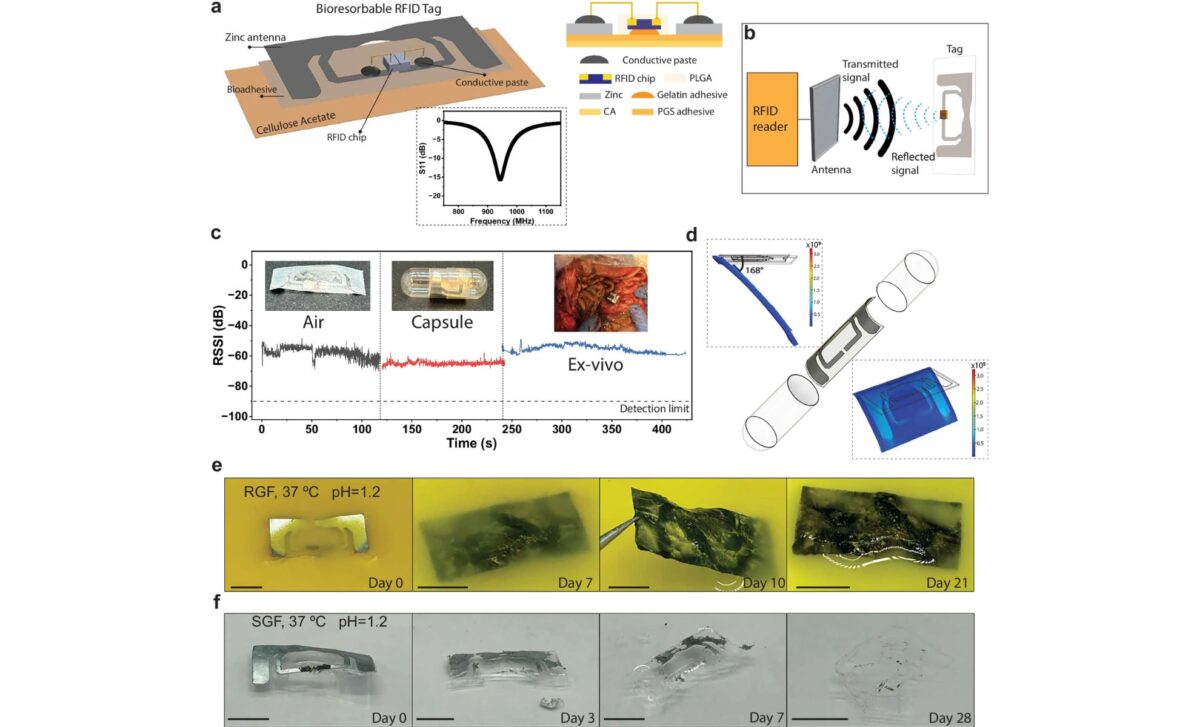
In swine models, the capsule successfully transmitted its signal from inside the stomach to an external receiver, even when the tag was floating in gastric fluid. As shown in in vivo imaging and tracking tests, the antenna remained operational for long enough to confirm ingestion, and then safely dissolved without causing blockage or harm.
According to the SAFARI project (Smart Adherence via FARaday cage And Resorbable Ingestible) the ultimate goal is to offer a passive, hands-off way to log ingestion events that could then be linked to patient health records. The capsule design is also compatible with existing gelatin or HPMC capsules, which streamlines integration into pharmaceutical workflows.
Eco-Conscious Materials and Minimal Health Risks
Beyond its clinical usefulness, the MIT capsule addresses growing concerns about medical waste and environmental sustainability. Current ingestible electronics often use non-degradable materials, which accumulate in the gastrointestinal tract or contribute to electronic waste.
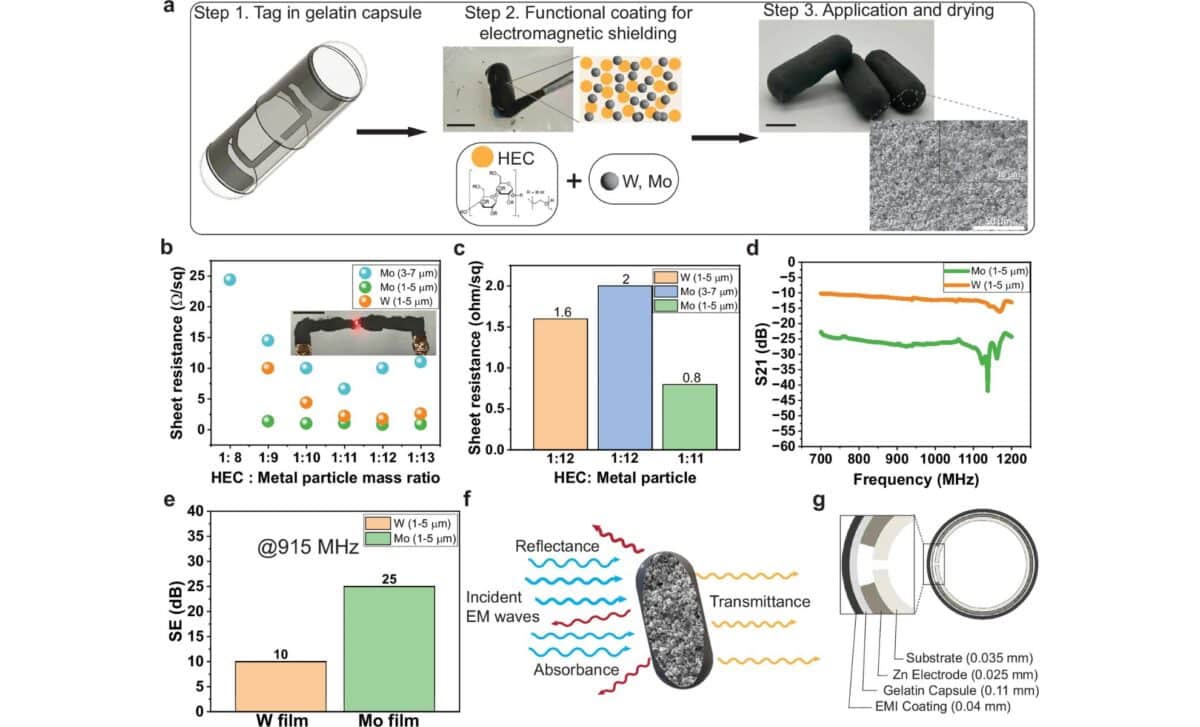
In contrast, the SAFARI capsule uses zinc and cellulose (both biodegradable and already approved for use in healthcare (along with a dissolvable protective coating. The team reports that the zinc antenna degrades at a rate of 120 nanometers per day, and Mo-based shielding disintegrates within hours after exposure to gastric fluids.
Data from blood serum tests in animal studies showed no significant increase in zinc or molybdenum levels post-ingestion. According to the MIT researchers, both elements are essential trace nutrients, and their quantities in the device are far below known toxicity thresholds. This makes the capsule not only safe for repeated use but also well-positioned for broader clinical trials.
The researchers are now preparing for human trials and envision wearable or portable receivers that could be integrated into daily routines, offering healthcare providers near-instant feedback on whether a dose was taken, without requiring any extra effort from the patient.
