Resurgence of the New World screwworm in Central America and Mexico has U.S. livestock producers on edge. The screwworm, a parasite, feeds on living tissue in livestock and other mammals.
NC State University entomologist Maxwell Scott, an expert on the screwworm, has studied the blowfly’s biology and helped map its genome. The goal is to identify genes that can be used to develop biology-based solutions to control screwworm that are more effective and less expensive.
Inside a clear container in the Scott lab, female Australian sheep blowflies lay their eggs in a tiny tent of raw hamburger meat. “They prefer the 93% lean,” Scott notes. These metallic-green blowflies are close relatives of the New World screwworm, making them ideal models for genetic research. Scott has worked with sheep blowflies in his research in New Zealand.
After moving to NC State in 2010, Scott became interested in transferring genetic tools developed for manipulating the sheep blowfly to the screwworm. We asked him to provide insight on efforts to eradicate the New World screwworm and explain new genetic approaches under development to control the parasite.
Can you describe New World screwworm in terms of the damage it causes and the threat it poses?
The New World screwworm is a blowfly. It looks very similar to some of the blowflies we have in North Carolina. The difference is the blowflies we have in North Carolina lay their eggs in dead animals and the larvae feed on the dead animal. So they’re important ecologically as decomposers or recyclers.
The screwworm, like some other blowflies, has evolved a parasitic lifestyle and feeds on living animals. Females seek out a live animal — usually a mammal, rarely a bird or other vertebrate — and look for any sort of opening, like a cut or a wound. If a female doesn’t find that, it’ll lay its eggs in and around other openings, such as the nasal cavity or ears.
When the eggs hatch, the larvae grow really quickly. They complete early development, or embryogenesis, in about six to seven hours, and then the larvae start eating, and they go through three stages. By the third stage, they’re quite big maggots, about two-thirds of an inch long. And they really burrow into the flesh. They’re difficult to get out. They have little hooks on them, so they’re called the screwworm because that’s what people see.
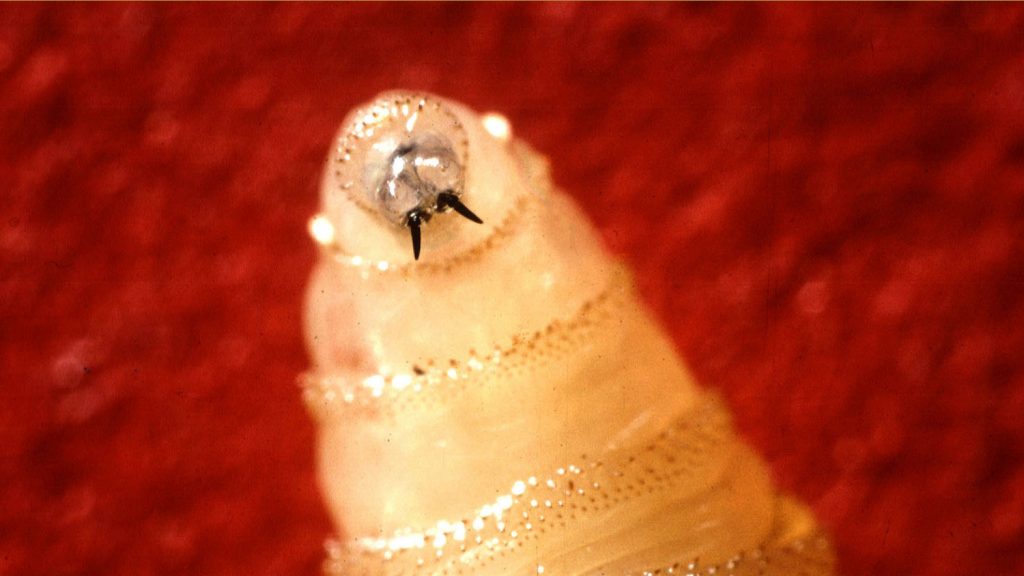 This close-up image of a New World screwworm larva shows its tusklike mouthparts and the ridges on its surface that resemble threads on a screw. Screwworm larvae feed on living tissue in livestock and other mammals. Photo: U.S. Department of Agriculture’s Agricultural Research Service
This close-up image of a New World screwworm larva shows its tusklike mouthparts and the ridges on its surface that resemble threads on a screw. Screwworm larvae feed on living tissue in livestock and other mammals. Photo: U.S. Department of Agriculture’s Agricultural Research Service
What kind of harm can the New World screwworm cause in livestock?
Screwworms in the flesh of the infested animals will cause a lot of damage to the area of the wound, which will make the animal ill. And if it’s not treated, what happens is the wound gets bigger as other flies come in and lay eggs, not just primary screwworm, and eventually they’ll kill the animal. Losses are significant. Even if the animal survives, there’s usually extensive damage to the hide and to the health of the animal. This is a multibillion-dollar pest in South America.
How long has it been since we’ve dealt with screwworm in a major way in North America?
Screwworm was declared eradicated from the United States in the 1960s, but there was still quite a large population in Mexico, so it may not have been residing in the U.S. but every year it came back. And depending on the winds and the weather, sometimes it came back pretty badly. There were many thousands of cases in 1972.
I know this because in 1976 the Entomological Society of America had a debate on eradication of insect pests versus pursuing other approaches like integrated pest management, and the test case was screwworm. We go through that debate in my genetic pest management graduate class, as it’s still relevant. The other reason we teach this is because it was the first demonstration of using a genetic approach to control a species.
The U.S. Department of Agriculture [USDA] extended the eradication campaign, which started in Florida in the 1950s, through Mexico. It took a long time, until the end of the 1980s or early ‘90s, before screwworm in Mexico was declared eradicated. After that, the campaign moved on through Central America, and by 2006 the screwworm was declared eradicated through Panama, to the Colombian border.
What was the genetic approach to eradicating the screwworm?
The genetic approach that the USDA conceived of was rearing flies in large numbers, sterilizing them and releasing them. They used mathematics — population genetic models — to predict how many flies would need to be released per area.
Insects were reared in a factory and made 100% sterile, but still fit to compete once they were released. The sterile males that were released mated with the fertile females out there. Under field conditions, the screwworm females only mated once — with a sterile male that didn’t produce any offspring.
The eradication was done with a massive facility in Mexico that produced 500 million sterile screwworm flies per week. It was later shut down for economic reasons and a plant in Panama produced about 15 to 20 million sterile flies per week to maintain a boundary at the Panama-Colombia border.
It was successful for 20 years or more. For reasons still not clear, the boundary failed and cases started appearing across Panama, eventually moving north through the rest of Central America. The movement across Central America was very rapid, which couldn’t have been the fly by itself. I think people were clearly not cooperating and shipping infested livestock. The Panama plant is now at full capacity of around 100 million flies per week to control the outbreak.
How does your lab use biotechnology to enhance the sterile insect technique?
We’ve engineered a gene that is switched off by adding an antibiotic called tetracycline to the blowflies’ diet. When we put them on a normal diet without the antibiotic, the gene becomes active and it kills female blowflies. Genetic suppression is much more efficient if only sterile male blowflies are released.
That’s what we worked on for a number of years, developing what we call male-only screwworm strains in Panama. With initial funding, I recruited a postdoctoral researcher who had worked in my lab in New Zealand. She was from Chile originally and happy to work in Panama, and she made really rapid progress. We had developed all the genetic technology using the sheep blowfly in my lab in New Zealand. We were able to rapidly transfer the technology to screwworm and produced male-only strains.
After that, we were funded to develop a second-generation system where the females died very, very early in development before they became larvae. Feeding the larvae is a big cost for factories producing so many insects to release, so it’s a significant cost savings. With the first generation system, the females didn’t die until the late larval or pupal stage. The female embryo lethal system was more difficult to engineer, but we did succeed in making some strains that look very promising. There is interest in obtaining regulatory approval to field test one or more of these strains.
You’ve also done some work using gene drives that “drive” a gene through a fly population. How does that work?
With a gene drive, we try to bias inheritance in favor of the gene we’re interested in — for example, that male-only gene. If a male is released carrying one copy of the gene, 50% of their offspring will inherit it and 50% will not. However, if we can couple the gene to a gene drive, then up to 90-100% inherit the male-only gene, and only 0-10% don’t. It becomes a much more powerful system for suppressing screwworm populations.
Again, this is where the math kicks in. So instead of having to release these huge numbers of insects that are needed for the sterile insect approach, you can release far fewer insects and achieve genetic suppression with the gene drive. It’s challenging, but one of the things we did with the USDA was assemble the screwworm genome. We released an initial genome map in 2020 and a much-improved one in 2022, and the USDA just released a third version in 2025. It’s a useful resource for everyone working on screwworm, but having an accurate genome makes it easier for us to get all the information we need to build a gene drive. One thing we’re interested in is coupling the male-only system to a gene drive.
Your lab here at NC State used CRISPR-Cas9 gene editing technology to develop the first gene drive in spotted-wing drosophila, an invasive fruit fly. What did that involve?
Another way to achieve suppression is just to target a gene that’s essential in females with a gene drive so that it is preferentially inherited. And if that gene stops females from developing but doesn’t affect males, that also becomes really effective. And we have built that in a different fly called spotted wing drosophila, which is an invasive pest in the Americas. The flies lay their eggs in small fruits like blueberries and strawberries before they ripen. That was one of the major papers from this lab since I arrived at NC State. It came out a couple of years ago in Proceedings of the National Academy of Sciences U.S.A.
We are now collaborating with scientists at the Institut Pasteur de Montevideo in Uruguay to develop a similar gene drive in screwworm. Developing very efficient gene drives is quite challenging, but I’m confident we will succeed as we have done for spotted wing drosophila.
For updates on the U.S. response to New World screwworm, visit the USDA Animal and Plant Health Inspection Service site:
New World Screwworm Emergency Response
https://www.aphis.usda.gov/animal-emergencies/nws
