Pressure-tuned optical properties
The strong excitonic effects dominate the optoelectronic properties of AgSePh at room temperature. To reveal the multiquantum-well features that govern the excitonic properties, we begin by exploring the pressure response of luminescence properties. As shown in Fig. 1a, the AgSePh crystals exhibit a narrow blue emission centered at 467 nm with a full width at half-maximum (FWHM) of 16 nm, attributed to the intralayer FEs confined within the 2D Ag-Se planes4,23. Meanwhile, we observe an ultraweak mid-gap broadband emission (Supplementary Fig. S1), assigned to the STE emission due to strong exciton-phonon interactions or defect-bound exciton emission4,23,35. Upon compression, the narrow excitonic emission slowly blue shifts with decreasing intensity, while the broadband emission maintains a constant intensity level. With the disappearance of the blue excitonic emission at 2.0 GPa, a sudden enhancement of the broadband emission occurs with an emission center shifting to 680 nm, indicating the efficient radiative recombination channels for the STEs were activated, followed by a sharp increase in intensity with the further increase of pressure. After the applied pressure exceeds 4.6 GPa, the emission entered a gradually weakening process until it disappeared at 8.3 GPa. Remarkably, the photoluminescence (PL) intensity has achieved a 27-fold increase at 4.6 GPa compared to its initial state at ambient conditions (Fig. 1c). The broadband emission exhibits a continuous blue shift behavior upon lattice compression, corresponding to a reduced FWHM process, which is much more pronounced than that of the narrow emission (Supplementary Fig. S4). A series of PL micrographs of a single crystal in the sample chamber clearly show the luminesce color and intensity transformations from blue to bright orange-red as a function of pressure (Fig. 1b). Large responses of excitonic properties under relatively mild pressure suggested that lattice contraction can effectively regulate the light emission behavior of 2D MOC AgSePh.
Fig. 1: Pressure effects on PL property.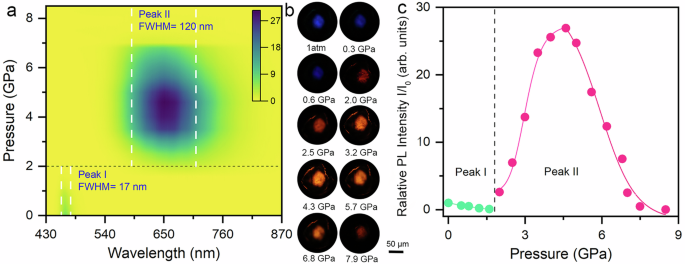
a Two-dimensional projection of the pressure-dependent PL emission spectra for AgSePh crystals. The black dashed line indicates the pressure value of emission transition. The white dashed line represents the FWHM of the emission spectra. b PL micrographs of AgSePh single crystal under different pressures. c Pressure-dependent relative PL intensity evolution of AgSePh. I0 represents the initial PL intensity.
To trace the variations of the electronic band gap, in situ high-pressure UV-Vis absorption spectra were carried out with the applied pressure up to 9.0 GPa (Fig. 2a). At ambient conditions, the AgSePh crystal displayed a steep absorption edge at about 480 nm, derived from the lowest energy excitonic transition4,20. The absorption edge first undergoes a slight blue shift up to 3.0 GPa, consistent with the evolution of FE emission under high pressure. Upon further compression, the absorption edge showed a significant red shift, while a new absorption edge emerged. The abrupt changes in steady-state optical properties imply the emergence of structural phase transitions at around 3.0 GPa, which significantly alter the electronic landscape and photogenerated carrier excited-states36,37. In the entire compression process, sub-band absorption was not observed, which excludes the contribution of the permanent defect states in AgSePh crystals. The pressure-driven band gap evolution of AgSePh is plotted in Fig. 2c. At ambient pressure, the band gap of AgSePh was determined to be 2.62 eV, corresponding to its bright-yellow color. Upon compression, the band gap initially increased slightly but then decreased dramatically, which is contrary to the trend observed in 2D organic-inorganic hybrid perovskites38,39. This confirms the uniqueness of the electronic structure and properties of MOC, despite their similar structural configurations. The minimum band gap we obtained is 2.34 eV at 9 GPa, which reflects a considerable piezochromic effect from bright-yellow to orange (Fig. 2b). To understand the composition of the band edge and the feature of the band gap, we investigated the electronic band structure of AgSePh by performing density functional theory (DFT) calculations. The calculated result suggested that AgSePh is a direct band gap semiconductor at the G point and possesses relatively large band dispersion, coinciding with the previous calculations (Fig. 2d)19,22,23. The band edges of the electronic band structure are dominated by contributions from the Ag-Se inorganic monolayer with low dispersion in the out-of-plane direction, corresponding to its multiquantum-well structures. The conduction band minimum (CBM) of AgSePh is mainly derived from Ag s orbitals, Ag p-orbitals, and Se p-orbitals, while the valence band maximum (VBM) is composed of Ag d-orbitals and Se p-orbitals (Supplementary Fig. S5). The conduction band also contains some orbital contributions from C p-orbitals. Therefore, the band gap evolutions as a function of pressure are controlled by Ag and Se orbital interactions, reflected both in the bond length and bond angle40,41. The bond contraction increases the orbital overlap between Ag and Se atoms, thereby increasing electronic band dispersion and narrowing band gap. In contrast, depending on the orientation and symmetry of orbitals within the Ag-Se inorganic monolayers, the enhanced intralayer distortion controlled by bond angle will decrease orbital overlap and band dispersion. The competitive nature of these two influencing factors determines the trend of the band gap under high pressure.
Fig. 2: High-pressure tuning of the band gap for AgSePh crystals.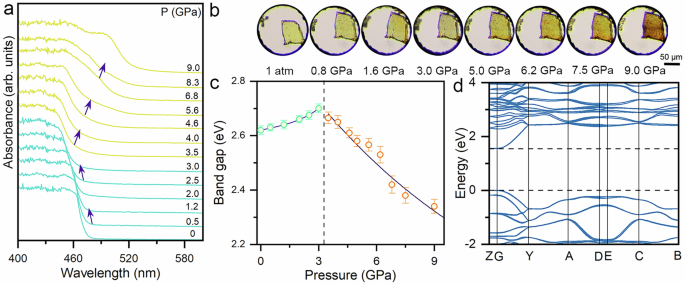
a Absorption spectra for AgSePh as a function of pressure. Purple arrows indicate the evolution of the absorption edge upon compression. b Optical micrographs of AgSePh single crystal under different pressures. c Pressure-dependent band gap of AgSePh. The error bars were obtained from the Tauc plot of the absorption spectra. d The electronic band structure for AgSePh with P 21/c space group.
Structural features and argentophilicity-mediated anisotropic compression
The specific multiquantum well heterostructures are responsible for the strong excitonic effects observed in AgSePh. To establish a foundation for comprehending the intricate connections between optical property transformations and lattice contraction, we conducted in situ high-pressure powder X-ray diffraction (PXRD) measurements at room temperature. The selected XRD patterns of AgSePh as a function of pressure are shown in Supplementary Fig. S8. As the pressure increases, all XRD peaks shift towards higher 2θ angles while maintaining their profile due to lattice contraction. The abrupt emergence of new peaks and discontinuous shifts of the original peak (8.4°) indicate a structural phase transition at 2.0 GPa42,43. Concurrently, the resolution and intensity of diffraction patterns at high 2θ angle ranges are noticeably reduced, which should be ascribed to the severe lattice distortion and reduced crystallinity caused by symmetry breaking and inhomogeneous deviatoric stress distribution44,45. We observed the onset of structural amorphization when the applied pressures exceeded ca. 12.0 GPa. As the pressure further increases, the amorphization degree of the samples gradually increases until the material transforms into a completely amorphous phase at 30.0 GPa, as reflected by the disappearance of all diffraction peaks, which corresponds to a highly distorted crystal structure with no long-range order. Upon decompression, AgSePh always remains in an amorphous state, indicating that the pressure-treated structure is irreversible (Supplementary Fig. S9).
The organic-inorganic hybrid semiconductor AgSePh adopts a layer-stacked multiquantum-well structure consisting of 2D sandwich layers bound together by interlayer van der Waals interactions (Fig. 3a). The atomically thin Ag-Se inorganic semiconducting layer is separated by the organic layer of benzene molecules that are covalently bonded to the Se atoms, with a 1.45 nm interlayer spacing between neighboring Ag-Se layers. The reported AgSePh crystallizes in the monoclinic centrosymmetric C2/c or P21/c space group at ambient conditions17,20,46, which have highly similar cell parameters and PXRD patterns (Supplementary Fig. S10). The relative rotation of the phenyl ring leads to a lack of long-range order arrangement, thereby affecting the symmetry of the structure. The disordered benzene rings present a linear arrangement in C2/c, while the ordered benzene rings exhibit a herringbone arrangement in P21/c (Supplementary Fig. S11)22,46. To systematically investigate pressure-induced structural evolution, we initially determined the ambient-pressure single-crystal X-ray diffraction (SCXRD) structure of AgSePh, revealing a monoclinic P21/c space group with a good structure solution (R₁ = 0.032). The Ag atoms exhibit coordinated tetrahedrally by four Se atoms and arranged into a distorted hexagonal pattern, leading to unusual in-plane anisotropy and three different Ag-Ag bond lengths, (Ag-Ag)1 = 2.95 Å, (Ag-Ag)2 = 3.09 Å, and (Ag-Ag)3 = 3.04 Å. The contact distances of Ag-Ag bond lengths are shorter than the sum of the van der Waals radii of two Ag atoms (3.44 Å), suggesting strong argentophilic interactions within the honeycomb-like Ag-containing layers3,17,19. Pawley refinement of the diffraction pattern at 2.0 GPa indicated that the high-pressure phase of AgSePh most likely adopts a monoclinic space group Cc with a lower structural symmetry (Supplementary Fig. S12). The structural phase transition mainly resulted from the in-plane anisotropic distortion in the Ag-Se layer. The pressure dependence of the lattice parameter is illustrated in Fig. 3b, where the discontinuous changes are consistent with the structural phase transition observed at 2.0 GPa. The layered AgSePh exhibits strong anisotropic compressibility in different directions, despite the fact that the applied pressure is isotropic. In the initial phase, the a axis exhibits the maximum compressibility in the in-plane direction, while the c axis exhibits intermediate compressibility in the out-of-plane direction. However, the compressibility of the inorganic in-plane a and b axes is comparable and much greater than that of the c axis with a significantly reduced compressibility in the high-pressure phase, despite the two phases having similar structural configurations and network connectivity. Dramatically, the inorganic intralayer compressibility of AgSePh is always greater than the interlayer compressibility in the out-of-plane direction throughout the entire compression process. This unique behavior differs from the two-step compression nature of the common 2D organic-inorganic hybrid perovskites and transition metal chalcogenides that the layer-to-layer contraction dominates the volume contraction in the out-of-plane direction at low pressures, and the near isotropic compression in the high-pressure range38,47,48. Flexible organic molecules and weak van der Waals interactions between adjacent layers in the layered AgSePh do not exhibit the expected maximum compressibility advantage in the out-of-plane direction, indicating the abnormal flexible nature of the in-plane inorganic layer.
Fig. 3: Structural evolution of AgSePh under high pressure.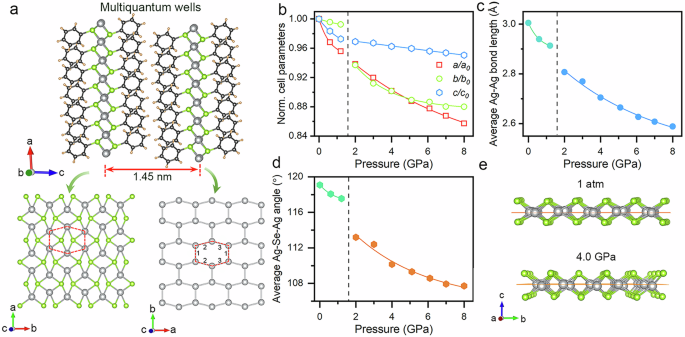
a The multilayered crystal structure of AgSePh. Gray ball: Ag; green ball: Se; black ball: C; orange ball: H. The Ag-Se inorganic monolayer viewed along the c axis, displays a distorted hexagonal lattice of in-plane Ag atoms (lower right). The argentophilic monolayer in the P21/c crystal structure with three different Ag-Ag bond lengths marked by 1, 2 and 3. (lower left). b The lattice parameter evolutions of AgSePh as a function of pressure. c Average length changes of Ag-Ag bond as a function of pressure. d Pressure-driven evolution of the average Ag-Se-Ag bond angle. e Ag-Se inorganic framework at ambient conditions and 4.0 GPa, displays the displacement of the Ag atom in the out-of-plane direction.
The unique compression behavior, wherein the intralayer structures are much easier to deform than that of the organic-inorganic hybrid interlayer structures in the out-of-plane direction, is unexpected. The high flexibility and low-density of the crystal structure serve as a critical physical foundation for achieving the anomalous pressure-dependence features49. The pressure-dependence of the unit cell volumes can be fitted by a second-order Birch-Murnaghan equation of state, yielding a bulk modulus K0 value of 12.54 GPa for the initial phase and 18.46 GPa for the high-pressure phase (Supplementary Fig. S14). Although pressure-induced lattice stiffening was observed, the small K0 value for both phases confirmed the soft nature of the lattice during the whole compression process. To qualitatively assess the structural evolution trend, we adopted the average bond angle and bond length changes by combining experimental cell parameters and DFT-based geometric optimization. The weakly attractive argentophilic interactions determine the strong compressibility of the Ag-containing intralayer structure in the in-plane directions, evidenced by the considerable contraction of the Ag-Ag bond lengths (Fig. 3c)27,29. Upon compression, the average Ag-Se-Ag bond angles are reduced, indicating the relative displacement of Se atoms away from the argentophilic monolayer in the out-of-plane direction (Fig. 3d). This behavior is further reflected by the fact that the compressibility of the Ag-Se bond is significantly lower than that of the weakly bound Ag-Ag bond, despite the Ag-Se bond length being positively compressed as a function of pressure (Supplementary Figs. S15, S16). The argentophilic interactions translate large positive area compression in Ag-containing layers into vertical internal strain, thereby balancing the external pressure in the out-of-plane direction (Supplementary Fig. S17), resulting in relatively low compressibility in the out-of-plane direction29. Compared to the coplanar Ag atoms in the initial phase, strong intralayer compression results in the obvious displacement of Ag atoms away from the coplanarity, accompanied by the variation in geometry of Ag…Ag interactions in the argentophilic layers (Fig. 3e). This implies that the argentophilic interactions help drive the structural phase transition with severe lattice distortion29. The mechanism underlying abnormal anisotropic compression in AgSePh is strongly associated with the variations in the Ag configuration environment. The impulsive vibrational spectroscopy and density functional perturbation theory calculations reported previously indicate that Ag atomic motions in the out-of-plane direction and Ag-Se interatomic bond spacing strongly couple to the excitonic transitions and exciton-phonon interaction19,34. The anisotropic compression dominated by the argentophilic interaction enhances the distortion of the inorganic framework layer, accompanied by the displacement of Ag and Se atoms in the out-of-plane direction. The enhanced local distortion within the inorganic layer contributes to the generation of large transient lattice deformations in the excited state, accompanied by stronger exciton-phonon coupling45,50. Moreover, the structural distortion modifies the electronic structure of the inorganic layer, which not only impedes charge carrier transport but also generates localized electronic states that significantly strengthen exciton-phonon coupling51. Therefore, the pressure-derived displacements of Ag atoms within the Ag-Se inorganic layer inevitably affect the light emission properties by modifying the electronic structure and exciton-phonon interactions.
The excitonic properties strongly couple to lattice phonons in flexible hybrid materials. To investigate the alterations in vibrational modes and the local coordination environment of Ag atoms, we carried out high-pressure Raman experiments at room temperature. At ambient conditions, we observe three dominant vibrational modes (61 cm−1, 88 cm−1 and 195 cm−1) in low-frequency ranges, which are identified as intrinsic modes of AgSePh associated with the inorganic sublattice (Supplementary Fig. S18)4,19,21,34. The stiffening of the vibrational modes (−1) under low temperature suggests that these resonances are related to the atomic motion within inorganic frameworks, which are linked to the excitonic optical transitions and light emission process of AgSePh34. The higher frequency vibrational modes are assigned to vibrations, rotations, and stretching of the phenyl component, which are not considered to have direct significant impact on the electronic structure. Upon compression, the low-frequency vibration modes blue shifted with weakened intensity due to lattice contraction (Supplementary Fig. S19). With the applied pressure up to 3.2 GPa, two new vibrational modes appear at 200 cm−1 and 303 cm−1, corresponding to the structural phase transition, and their intensity clearly enhances with increasing pressure. The early results suggested that the vibrational mode (∼200 cm−1) might be the one dominating the absorption and emission properties because it participates in the exciton scattering process34. In addition, we noticed a new weak vibrational mode at 103 cm−1 at 4.5 GPa, which cannot be clearly resolved due to the high background Raman signal generated by the Rayleigh wing19,34. This vibrational mode was assigned to a mixed phonon mode, involving the wagging of adjacent phenyl rings and corresponding stretching of the Ag-Se bonds, which are associated with strong exciton-phonon coupling. The benzene ring is anchored to the Ag-Se inorganic layer via the C-Se covalent bond, making the C-Se bond a sensitive probe for monitoring alterations in the local Ag-Se coordination environment. Therefore, the significant change in C-Se stretching mode (303 cm−1) further reflects the local changes within the Ag-Se inorganic layer. On the contrary, the vibrational modes of organic components do not show obvious changes under high pressure, except for gradually decreasing intensity. When the pressure exceeded 9.1 GPa, all vibrational modes disappeared, stemming from the appearance of amorphization and disordered structures. The main phonon modes that strongly couple to the excitonic transitions showed considerable changes after the phase transition, suggesting the atomic displacement within the inorganic layer brought about a significant impact on exciton-phonon interactions.
Pressure-tuned exciton dynamics and photophysical mechanisms
To clarify the underlying mechanisms of the pressure-induced transformations in excitonic emission, in situ high-pressure time-resolved PL measurements for AgSePh were performed to investigate the decay dynamics of two emission peaks. As shown in Fig. 4a, the initial narrow emission of AgSePh crystals exhibited ultrafast decay dynamics, consistent with the previously reported time scale4,20,23. Upon compression, the PL decay was gradually prolonged, with the lifetime increasing from ∼400 ps at ambient pressure to 1.2 ns at 1.2 GPa, as determined by single-exponential function fitting (Fig. 4c). The ultrafast lifetime of the FE recombination is ascribed to the large binding energy (400 meV) and large oscillator strength4. The large Eb in layered AgSePh stems from two cooperative effects: quantum confinement and dielectric confinement50. The exciton’s wave function is confined into the 2D Ag-Se inorganic monolayers, resulting in quantum confinement with the increased Eb. The dielectric confinement is attributed to poor screening effects on the attractive interaction between electrons and holes due to the low dielectric constant of the organic layers compared to the inorganic layers, which further enhances Eb. Owing to the soft nature of the organic- inorganic self-assembled structure, the significant contraction of inter- and intralayer structures decreases the barrier potential and interlayer dielectric mismatch, thereby suppressing the quantum and dielectric confinement of the excitons in the 2D inorganic layers accompanied by a decreased Eb52,53,54. Therefore, the reduced exciton binding energy should be responsible for the prolonged lifetime of FEs under high pressure. Compared to the ultrafast dynamics of the narrow emission, the broadband emission exhibited a slow decay on the nanosecond scale, implying that it originates from radiative recombination of STEs (Fig. 4b and Supplementary Fig. S20)15,50. The PL decay of the broadband emission was well described by a tri-exponential function with two fast-components and a slow-component. The predominant slow-component is attributed to the recombination of STEs with increasing lifetime upon compression from 44 ns at 1.6 GPa to 188 ns at 4.0 GPa (Fig. 4d). Thereafter, the recombination lifetime of STEs decreased under higher pressure, in coincidence with the evolution trend of its PL intensity. The two fast-components are ascribed to the defect states originating from the pressure-induced phase transition and reduced crystallinity, which can act as fast nonradiative recombination centers to trap the excited charge carriers23,55.
Fig. 4: Carrier dynamics of AgSePh under high pressure.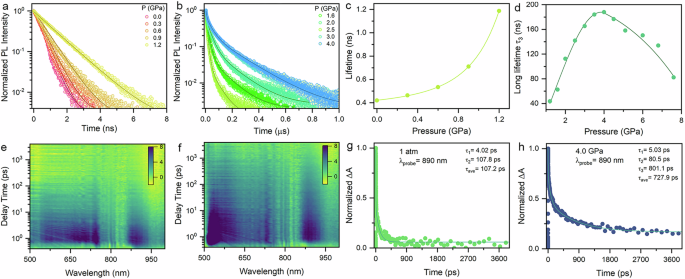
a TRPL spectra of high-energy narrow emission under high pressure. b TRPL spectra of low-energy broadband emission under high pressure. c Pressure-dependent carrier lifetime for high-energy narrow emission. d Pressure-dependent long lifetime τ3 for low-energy broadband emission. e Pseudocolor TA plot of AgSePh at ambient conditions. f Pseudocolor TA plot of AgSePh at 4.0 GPa. g PIA decay dynamics of AgSePh crystal at 1 atm, corresponding to a probe wavelength of 890 nm. h PIA decay dynamics of the AgSePh crystal at 4.0 GPa, corresponding to a probe wavelength of 890 nm.
To further elucidate the photophysical mechanisms of the broadband emission and ultrafast carrier dynamics process in AgSePh, we conducted femtosecond TA measurements. The pseudocolor TA plots of AgSePh crystals are shown in Fig. 4e, f at ambient conditions and 4.0 GPa with a wide probe range of 500-980 nm. Under the photoexcitation by a 378 nm laser, a broad positive photoinduced absorption (PIA) was observed across the entire probe region in AgSePh, directly confirming the existence of the transient STE states56,57. This feature strongly supported that the mid-gap broadband emission in both phases is attributed to the localized STE recombination rather than defect states. At ambient conditions, an ultrafast self-trapping lifetime (∼1 ps) was evaluated according to the rising process of the PIA signal at different wavelengths (Supplementary Fig. S22), confirming that there is nearly energy barrier-less separating the FEs and STEs23. A faster formation time (∼500 fs) of STE states was observed at 4 GPa, which should be ascribed to the strong exciton-phonon coupling and smaller or absent energy barrier58. The enhanced exciton-phonon interaction results in a significant population of stable STEs by preventing the STE detrapping back to the FE state at room temperature, corresponding to the absence of the narrow FE emission on PL spectra. Compared to the initial phase, on the one hand, the PIA intensity of AgSePh was significantly enhanced in the high-pressure phase, implying an increase in both the oscillator strength and density of the photogenerated STEs in the layered AgSePh crystals, thereby boosting the broadband STEs emission23,59. On the other hand, the PIA decay process of AgSePh in the high-pressure phase (τave ~ 728 ps) is obviously slower than that of the initial phase (τave ~ 107 ps), which is a common feature for “bright” STEs (Fig. 4g, h)58,60. The PIA decay curve of AgSePh at ambient conditions can be fitted by a biexponential function with an ultrafast component (τ1 ~ 4 ps) and a slow component (τ2 ~ 107 ps). The ultrafast component is attributed to the exciton-phonon scattering61,62. The slow component is assigned to the “dark” STE states in the AgSePh crystal56. At 4.0 GPa, the PIA decay curve of AgSePh can be fitted by a biexponential function with three components: an ultrafast lifetime of τ1 ~ 5 ps, a moderate lifetime of τ2 ~ 81 ps and a long lifetime of τ3 ~ 801 ps. The prolonged lifetime of the ultrafast component implies the suppressed carrier-phonon scattering in the high-pressure phase, which may also contribute to boost the STE emission19,62. The added fast component should be assigned to the carrier trap states associated with the lattice defects, which act as the nonradiative recombination centers and is responsible for emission quenching56,63. The long lifetime of τ3 ( ~ 801 ps) is ascribed to the “bright” STE states, which agrees well with the long-lived process of the broadband emission. The same PIA decay signals probed at different wavelengths suggested that the PIA signal of AgSePh stemmed from the same excited state (Supplementary Fig. S23)56. The results of TA demonstrate that the broadband emission originated from the STE states and enhanced STE populations by increasing the exciton-phonon coupling strength in the high-pressure phase. The high-pressure phase maintains identical band edge composition and direct band gap characteristics to the initial phase, confirming the intrinsic exciton emission nature (Supplementary Fig. S7).
The polaronic effect associated with the exciton-phonon interaction plays an important role in manipulating the intrinsic nature of the narrow or broadband emission in the hybrid AgSePh with soft and anharmonic lattices64. Excitons can couple to lattice vibrations through both long-range Fröhlich interaction (large polaron) or short-range Holstein interaction (small polaron)50,65. Large polarons involve more extended electron-phonon interactions across several cell units, dominated in polar and ionic materials, while small polarons arise from strong exciton-phonon coupling with self-induced transient lattice deformation within approximately a unit cell, dominated in soft materials featuring dynamic lattice distortion and anharmonic phonons. The interplay strength between exciton and phonon dictates the excited-state energy landscape and polaronic properties. We assign the narrow emission arises from the delocalized FE states (large polaron), while the mid-gap broadband emission is attributed to the localized STE stats (small polaron)35. Previous multiple evidence has confirmed that AgSePh is an edge case where the two polaron scenarios overlap with intermediate exciton-phonon coupling strength at ambient conditions34. In the initial phase, the momentarily trapped exciton forms metastable STEs by coupling with the lattice vibrations, thereby reaching thermodynamic equilibrium between FE states and STE states with a small activation energy (Supplementary Fig. S24). In the high-pressure phase, the large out-of-plane distortion of the inorganic layer stemming from easily deformable argentophilic interactions enhances the short-range exciton-phonon coupling strength, resulting in stable STEs in the excited state. The stable STEs cannot detrap and favor direct radiative recombination, corresponding to a broadband emission.
Single ensemble luminescence dynamics
The broadband emission with a large Stokes shift in semiconductors has commonly been attributed to intrinsic STEs, permanent lattice defects, indirect band gap recombination or extrinsic substitutional impurities50,66,67. The calculated electronic structure has excluded indirect bandgap recombination, which is consistent with earlier research. Doping with Te element can lead to broadband emission but we do not consider this behavior in pure AgSePh crystals15. Our steady-state and transient absorption spectra have provided evidence to exclude the broadband emission that originates from extrinsic defect states. To further elucidate the origin of broadband emission in AgSePh crystals, we constructed the time- dependent emission spectra by integrating PL decay curves at different wavelengths, where each decay was recorded under the same experimental conditions and integration time (Fig. 5a). If the lifetime and emission line shape of a portion of the time-dependent emission spectrum differs from those of the long-time spectrum, it cannot be considered as part of the same ensemble23,68. The time-resolved emission spectra present the invariant shapes and emission centers within a long decay period of time, confirming single ensemble emission and excluding other subensembles with different time scale decays in AgSePh crystals (Fig. 5b). Wavelength-independent PL decay curves imply single ensemble decay dynamics, while the excitons trapped in lattice defects exhibit wavelength-dependent PL decay dynamics due to distinct environment results in a series of self-trapped states (Fig. 5c). Although the structural phase transition produces a large number of lattice defects, this ensemble of defect sites brings about very fast nonradiative relaxation, which does not affect the emission spectrum, but produces two fast components associated with the defects in the PL decay kinetics68. Combined with the above comments, our multiple evidence suggests that the bright broadband emission in AgSePh stems from the intrinsic STEs with strong exciton-phonon coupling strength. Owing to the hybrid nature and soft lattice of AgSePh, its crystal structures and optical properties are extremely sensitive to external pressure, which is the same characteristic as that of other 2D van der Waals materials69,70. The blue shift trend of the FE emission in AgSePh under high pressure aligns with that observed in monolayer TMDs such as 1H-WS₂ and 1H-MoWS₂71. In contrast, 2D MHPs display distinct red-shifted FE emission upon compression39,52. Pressure engineering can effectively regulate STE emission in various van der Waals layered materials by manipulating lattice distortion and electron phonon interactions. Similar to AgSePh, AgTePh exhibits strong argentophilic interactions within their 2D Ag-Ag networks. In contrast, AgSPh displays linear Ag-Ag argentophilic interactions in its inorganic layer. The combined effects of argentophilic interactions and chalcogen composition variations can induce diverse anomalous compression behaviors and tunable optical properties through pressure engineering. The pressure-induced exciton emission transformation in AgSePh crystals show potential applications in pressure sensors, pressure switches, and information storage.
Fig. 5: Time-dependent emission spectra.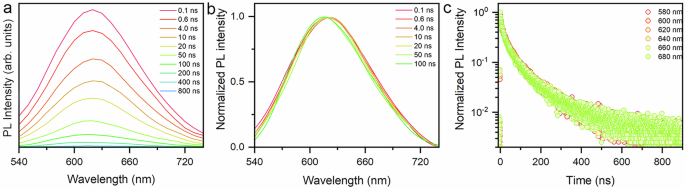
a Representative time-dependent emission spectra of AgSePh at 4.0 GPa. b Normalized time-dependent emission spectra of AgSePh at 4.0 GPa. c Wavelength-dependent PL decay for AgSePh at 4.0 GPa.