A deadly fungus found in ancient tombs could make for a promising anticancer drug.
Snake venom could be turned into lifesaving antibiotics.
And extracts from common trees hold promise as therapies for neurodegenerative diseases.
These are three ways that Philadelphia-area scientists aim to build on the long legacy of scientists who have successfully derived new medicines from natural products. Some estimates suggest that 40% of modern medicines come from plants, including the most popular prescription drugs.
Scientists would often find these medicines through trial-and-error or even happy accidents. The lifesaving antibiotic penicillin, for example, was identified after mold somehow contaminated a petri dish in 1928. The chemotherapy paclitaxel, used for multiple types of cancers, originated from the bark of the Pacific yew tree.
“Nature already made so much stuff for us to explore and then try to learn from,” said Xue (Sherry) Gao, an associate professor in the University of Pennsylvania’s school of engineering.
Now, better experimental tools and technological advances are changing the way a centuries-old approach to drug discovery is carried out.
Artificial intelligence has sped up the rate of discovery from years to hours in research conducted by Gao’s colleague at Penn Engineering, Cesar de la Fuente.
At Haverford College, a scenic campus arboretum has become a laboratory for biology professor Robert Fairman’s innovative methods for testing tree extracts in animal models and mapping out how they intercept disease processes.
These scientists see their task as figuring out what lifesaving therapeutics could be hiding in plain sight.
From toxic fungus to cancer drug
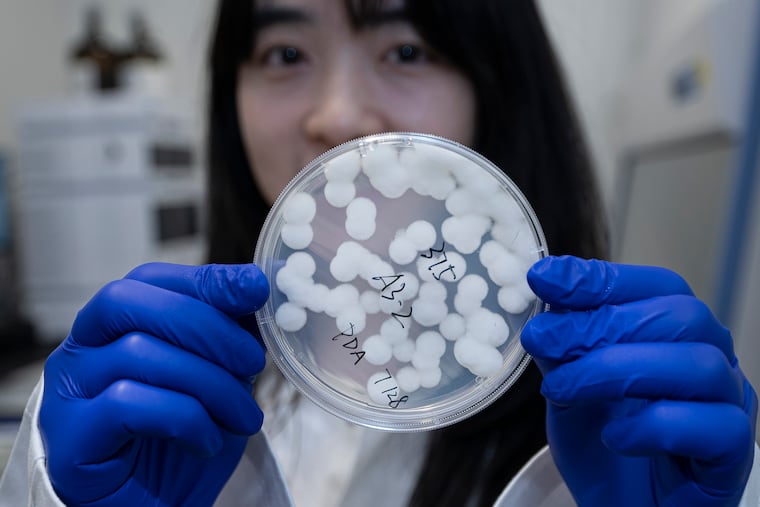
Aspergillus flavus doesn’t usually get good press.
This fungus is commonly (and perhaps erroneously) linked to the mythical “Pharaoh’s curse” associated with King Tutankhamun’s tomb, without strong evidence to support that narrative.
It is also thought to have caused deadly lung infections in several scientists who entered the tomb of King Casimir IV in the 1970s.
Now, thanks to Gao, its reputation may improve: Her lab turned the fungus into a leukemia-fighting agent.
Many scientists, including her, choose to study this strain of fungi because it’s so common in the environment. Her team started by growing the fungus and then searching for compounds they could harvest from it.
One stood out for its highly complex structure: a compound comprised of seven rings fused together. This type of molecule would generally be linear, Gao explained.
“Meaning it’s very unique,” she said.
It was a new class of compounds that hadn’t been described before. Curious if its abilities could be as special as its shape, they tested it against a variety of targets: bacteria, fungi, and cancer cells grown in the lab.
It proved potent against leukemia cells and had little effect on normal cells.
This desirable duality — deadly to cancer cells and gentle to healthy cells — is due to the compound being so big that it can’t enter cells freely. Instead, it needs to be let in by a transporter, a type of protein that acts much like a doorman at the entrance of a building.
Their theory is that leukemia cells contain this transporter at higher levels than normal cells, allowing more of the compound to enter and ultimately kill the cell.
Going a step beyond nature, the team engineered the compound to be even better at entering leukemia cells by adding a fatty molecule to it. This made it as effective as two FDA-approved leukemia drugs, cytarabine and daunorubicin, according to results published earlier this summer in the medical journal Nature Chemical Biology.
Most exploratory scientific efforts never advance to the point where they are found safe and effective for humans. The next step for this compound will be to test it in animals and eventually move into humans. Even if the results continue to be promising, it could be years before any potential new drugs become widely available to patients.
Many more great medicines are just waiting to be found in fungi, Gao believes. These organisms “are so clever to make really complicated molecules,” she said.
For the “majority of them, we don’t know what their potential even can be,” she added.
Snake venom as medicine
The search for new medicines in nature has traditionally amounted to looking for a needle in a haystack.
Scientists dig into dirt, fish samples from the ocean, and grab extracts from random plants.
“It’s a really painstaking process that can take many, many years, and at the end of the day, oftentimes, does not yield any good candidate,” said de la Fuente, a scientist at Penn’s school of engineering and medical school.
Several years ago, he started using AI to accelerate the process.
He trained algorithms in his lab to recognize qualities that make certain molecules more effective drugs. They search for key patterns at the DNA and protein level, much like a barcode reader scans for specific combinations of black and white bars.
Currently, he is searching for novel antibiotics in an unusual source: snake venom.
“Venoms are these evolutionary masterpieces that have evolved through millions of years,” de la Fuente said.
He instructed AI to examine venoms at the protein level and flag any portions that could be deadly to bacteria for the project. This yielded hundreds of candidates within hours.
Of those, researchers synthesized 58 in the lab, and found 53 were capable of killing at least one drug-resistant strain of bacteria, without harming human red blood cells.
Their work, which so far has been tested in laboratory models and not yet in humans, was described in a July article published in the medical journal Nature Communications.
“We are unveiling novel attributes of venoms that might be actually good for humanity,” he said.
In the years prior, de la Fuente’s lab similarly screened for antibiotic compounds across ancient biology, including the genomes of giant sloths, ancient penguins, and other creatures of the past.
His two most promising antibiotic candidates are named “neanderthalin,” found in the genome of Neanderthals, and “mammuthusin,” from the Woolly mammoth genome.
“We’re actually resurrecting those molecules because they no longer exist in the biological world around us,” he said.
Aging like a tree
At Haverford College, trees far outnumber students. The college campus doubles as both a place of study and a 216-acre arboretum filled with 5,000 trees.
Fairman, who has been teaching biology at Haverford for nearly three decades, is working to prove that these trees could be promising therapeutics for neurodegenerative diseases.
He was inspired to look at trees several years ago after reading a paper that showed that trees, which can survive up to thousands of years, are more effective at preventing protein buildup the longer they live.
Humans are the opposite. As we age, biological processes gradually become more error-prone, increasing the risk of clumping of misfolded proteins.
That aggregation is “the foundation of all these diseases,” Fairman said, referring to neurodegenerative conditions like Alzheimer’s, Parkinson’s, and Huntington’s disease.
Over the last few years, his lab has collected leaves, bark, roots, and the buds of branches from trees in the campus arboretum.
After grinding them up and processing them, his team feeds the extracts to disease-modeling animals used for scientific testing: from worms with Huntington’s disease to fruit flies with Parkinson’s disease.
When first testing extracts, his lab looks for a reduction in behaviors associated with the diseases. For example, he tests whether worms with Huntington’s disease, a condition that affects movement and cognition, have less trouble swimming. For the flies, he sees if it helps them sleep normally.
So far, they’ve found oak trees aren’t effective, but there are many extracts from red and sugar maple trees that inhibit Huntington’s and Alzheimer’s disease in animal models.
They’re collecting evidence now to see whether these extracts work by preventing protein aggregation.
Work still needs to be done to identify exactly which compounds within these extracts could be driving these effects.
“For the long lived trees, there’s probably many different compounds that can have that activity. So it would probably be a process to identify a whole set of them,” he said.