A total of 29 individuals with mitochondrial variants associated with LHON were identified in the ophthalmic genetics service. Six tested positive for the m.14484T>C variant and the remaining 23 tested positive for the m.11778G>A variant.
All patients were offered treatment, excluding those who had experienced prior spontaneous visual recovery or exhibited LHON with ‘plus’ features, such as one female patient with Harding’s syndrome. No restriction was placed on time from vision loss, although a number of those with the longest potential durations of disease were either not being routinely followed up, or had been lost to follow-up, or had been discharged some years before. Thus, 12 were initiated on idebenone therapy under the NHS ophthalmic genetics clinic in UHW in accordance with the AWMSG guidance. All were offered six-monthly appointments to monitor vision and any side effects. One patient was excluded from the current analysis as they were restarted on therapy for only 6 months, having previously been treated within the LEROS trial [13]. There were 8 males and 4 females, with an average age when initiating therapy of 47.2 (±19.3) years, ranging from 22 to 72 years of age. Prior to starting idebenone, the mean duration of symptoms attributed to LHON was 31.5 (±59.5) months with a total range from 2 to 216 months. Four patients tested positive for the m.14484T>C pathogenic variant, and the remaining eight carried the m.11778G>A variant. Two treated patients had a known family history of LHON, one with the m.14484T>C variant and one carried the m.11778G>A variant. The remaining 10 patients had no clear family history and were suspected de novo variants, albeit not all family members have undergone genetic testing to investigate their carrier status.
The NH cohort comprised 346 eyes reaching 12 months of follow-up and 168 eyes achieving 24 months of follow-up. NH cohort patients had an average age of 31.7 (±15.0) years for those achieving 12 months of follow-up and 29.7 (±14.8) years for those achieving 24 months of follow-up. These were both significantly different from the treated patients in the current cohort (p p = 0.134; 24 months v treated, p = 0.287). In terms of the proportion of patients in the subacute/dynamic phase of the disease versus the chronic phase of the disease (as defined previously as 1 year for chronic) [13], this was not significantly different from the current treated Welsh cohort in comparison to the NH cohort at either the 12-month time point after starting treatment/follow-up (idebenone 56% v NH 56%, p = 1.00) or the 24-month time point after starting treatment/follow-up (idebenone 57% v NH 45%, p = 0.412).
The cohort treated with idebenone had a mixed history of alcohol and tobacco usage. Six reported smoking more than 10 cigarettes a day, although two of these subsequently ceased smoking after their diagnosis of LHON. Four of the cohort reported drinking 10 or more units of alcohol per week, with two reporting heavy use of 40 units or more. Of the entire cohort, no mention was made of significant tobacco usage or alcohol consumption in the case of three individuals, whereas two individuals reported zero tobacco smoking or alcohol consumption.
At the point of data collection, the patients had received an average of 30.2 (±9.9) months of therapy, ranging from 14 to 42 months in total. No side effects were reported. Two patients were excluded at this point of analysis due to being lost to follow-up, leaving 10 patients continuing therapy. Additionally, one patient died whilst taking idebenone of coexistent medical comorbidities after 13 months of therapy. Two patients had ceased therapy after 30 and 35 months, respectively, by mutual consent on clinical review. One ceased due to no response over the 30 months of therapy. The second individual demonstrated CRR between 21 and 30 months; however, by 35 months, their vision deteriorated to baseline in both eyes and they elected to cease idebenone therapy.
The primary outcome of the study was visual acuity, with patients having a mean visual acuity equivalent to 1.89 (±0.71, 24 eyes) LogMAR units at diagnosis of LHON. At the time of therapy initiation (0 months), the patient’s vision had deteriorated to 2.22 (±0.32, 24 eyes) LogMAR units. The most recent visual acuity for the 7 patients still receiving idebenone therapy is 1.69 (±0.72, 14 eyes) LogMAR units. The peak mean visual acuity within the first 36 months of therapy was at 27 months, which was 1.12 (± 0.77, 8 eyes) LogMAR units for the limited patients with a review at this time point. The time course of visual acuity is illustrated in Figs. 1 and 2.
Fig. 1: The mean visual acuity (LogMAR) of 12 patients with LHON treated with idebenone over time (months).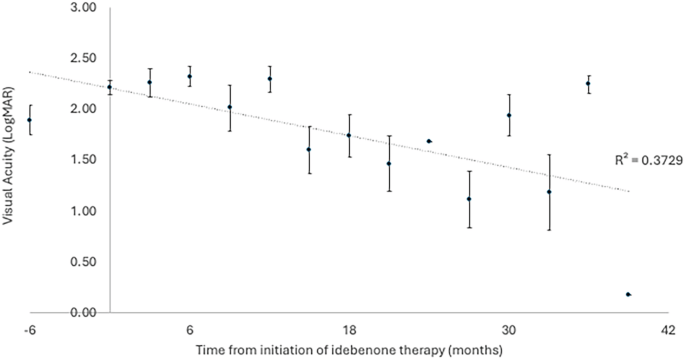
Displayed as mean ± standard error of the mean.
Fig. 2: The measured visual acuity (LogMAR) of each eye in all 12 patients treated with idebenone over time (months).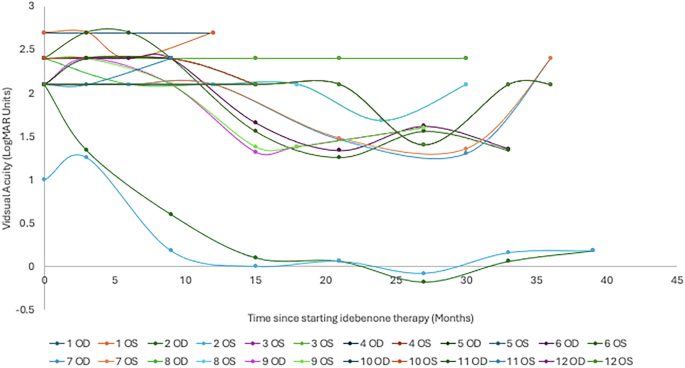
OD—right eye; OS—left eye.
In line with the previously published LEROS study [13], comparison was made between visual acuity outcomes at 0 months, 12 months and 24 months of therapy. There was no difference between 0 months (2.22 ± 0.32) and 12 months (2.11 ± 0.62; p > 0.05). Whereas there was significant improvement in vision after 24 months of therapy (1.48 ± 0.72) from both 0 months of therapy (Bonferroni adjusted p = 0.042) and 12 months of therapy (Bonferroni adjusted p = 0.004).
In terms of CRR, 10% of a total of 10 patients (20 eyes) reaching 3 months of therapy demonstrated CRR. This increased to a peak of 86% of 7 patients (14 eyes) reaching 27 months of therapy, but declined thereafter with far fewer patients (3 patients, 6 eyes) reaching beyond 34 months of therapy. This is outlined in Fig. 3.
Fig. 3: Number of eyes within the cohort of patients treated with idebenone demonstrating clinically relevant recovery (CRR) at each time point (months).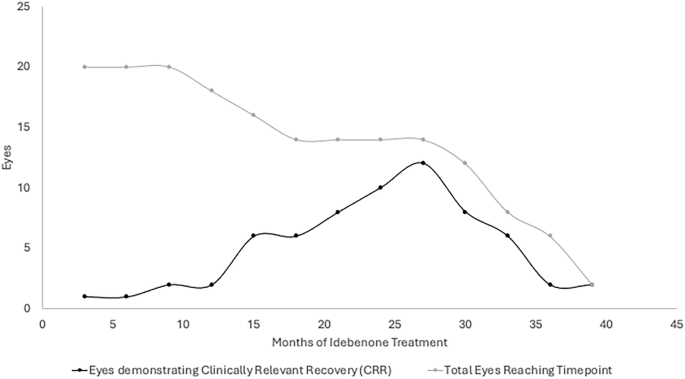
The total number of eyes within the cohort that reach each time point after initiation is represented to demonstrate the relative proportion of eyes reaching a time point that demonstrate CRR. CRR is defined as improvement in vision from ‘off-chart’ to at least 1 letter ‘on-chart’, or improvement of at least 10 letters ‘on-chart’.
The 12- and 24-month CRR outcomes were compared with the NH cohort. At 12 months, there was no significant difference in CRR rates between the treated group and the NH group (idebenone 11% v NH 19%; p = 0.545). However, at 24 months, CRR was significantly higher in the Welsh cohort treated with idebenone compared to the NH cohort (idebenone 71% v NH 24%; p χ2 (3) = 29.243, p R2) and predicting 76.9% of cases correctly. Both treatment with idebenone (OR 0.105, 95% CI 0.021 to 0.532; p = 0.008) and a younger age (OR 0.737, 95% CI 0.589 to 0.923; p = 0.006) were significant in contributing to this model; however, an individual’s sex was not (OR 1.117, 95% CI 0.454 to 2.748; p = 0.810).
The individual patient who demonstrated CRR at 3 months has continued to improve up until 27 months with a peak visual acuity of −0.18 LogMAR units in the right eye and −0.08 LogMAR units in the left eye. This has, however, deteriorated up to 39 months, where their vision was 0.18 LogMAR units in the right eye and 0.18 LogMAR units in the left eye. This is relative to a visual acuity of counting fingers (equivalent to 2.1 LogMAR units) in the right eye and 1.0 LogMAR units in the left eye at the point of initiating therapy.
Examining the visual field tests of this individual demonstrated some improvement in terms of visual field index (VFI) and mean deviation (MD) on Humphrey 24-2 visual field testing using the SITA standard protocol. There was only a full-field 120-point suprathreshold test available prior to treatment initiation, which demonstrated a centrocecal scotoma within the central 20 degrees of both eyes. At 9 months after therapy initiation, the individual achieved a VFI of 75% in the left eye and 59% in the right eye and a MD of −8.39 dB in the left eye and −13.00 dB in the right eye. After a further 13 months of therapy, at 22 months total, they achieved a VFI of 77% in the left eye and 66% in the right eye, as well as an MD of −7.50 dB in the left eye and −10.09 dB in the right eye.
Three cases were identified as developing vision loss with no previous visual symptoms in older male patients (63, 68 and 72 years of age, all from separate families) with the m.14484T>C pathogenic variant coincidentally subsequent to COVID-19 vaccines. All three of these individuals started on idebenone within 4–8 months of the onset of symptoms. All three also demonstrated CRR at some time point during their treatment between 15 and 33 months. However, at the most recent review, one had ceased therapy due to completing 36 months of treatment and not retaining CRR. Meanwhile, the others continue therapy, although one individual’s vision has returned to baseline and the other retains some vision at 1.36 and 1.34 LogMAR units in the right and left eyes, respectively.