This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two authors (MJP, MDC) performed an independent search in the PubMed database from January 1990 to November 2024, utilising the MeSH term “migraine with aura,” restricted to the English language. A total of 1164 records were identified. After screening the titles and abstracts for relevance, the authors (LJ, MJP, MDC, GB, RB, AA, DA, YS) excluded 584 records. Any conflicts were resolved through discussion and consensus among all participating authors. One hundred twenty-seven full articles were deemed eligible, and an additional 59 articles were added through cross-referencing. We excluded 455 articles for the following reasons: absence of abstract, age under 18 years, insufficient data, and irrelevance to the topic.
Epidemiology of migraine with aura
Estimates of MA occurrence in the general population range from 6.3% to 23%, influenced by factors such as genetics, gender, age, and geographic distribution [10,11,12]. The prevalence of migraine increases during puberty, peaks between the ages of 35 and 39, and then declines in later life, particularly after menopause in women [13]. Findings from the Heinz Nixdorf Recall Study, which examined a population-based cohort of 2,038 individuals aged 65 to 86 years, indicated that 9.4% of participants reported experiencing active migraine, with 3.5% specifically suffering from MA [14]. In line with broader epidemiological trends, a 2004 Danish cohort study applying ICHD-2 criteria to 362 patients reported a higher prevalence of migraine with aura among women [10].
Recent studies emphasize that migraine in men are often stigmatised due to their historical association with women, resulting in underreporting, inadequate treatment, and disparities in healthcare. Furthermore, research on gender diversity indicates that hormonal and societal factors affect migraine prevalence across different populations [15].
Significant geographic variations exist in the prevalence of MA. A multicenter study conducted in Japan using the ICHD-2 criteria demonstrated that migraine with aura affects approximately 6.3% of the general population [12]. Conversely, a study from Italy reported a notably lower prevalence of MA at 1.6%. In contrast, the prevalence of MA in the United States and Europe ranges from 12 to 18% among women and from 6 to 9% among men [16, 17]. A study in Mexico involving 1,147 migraine patients found that 53% had MA, a rate consistent with international data; however, women exhibited a higher prevalence with a 4:1 female-to-male ratio [18]. Additionally, research in Korea found that 29.4% of migraine patients experienced visual aura, a rate comparable to those in other populations [19].
Furthermore, the clinical presentation of MA evolves with age. While the intensity of headache tends to diminish over time, aura symptoms, particularly visual disturbances, may become more pronounced.
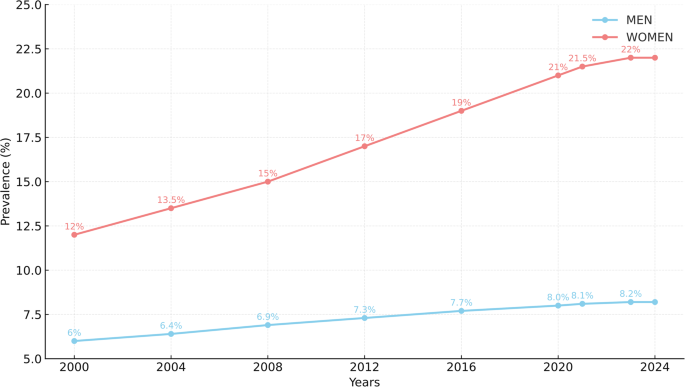
Figure 1 summarises the changes in the prevalence of MA from 2000 to 2024 [11, 17, 20,21,22].
Changes in the prevalence of MA from 2000 to 2024
Genetic basis of MA
Migraine is a disorder with a hereditary component. Compared to the general population, first-degree relatives of individuals with MO are 1.9 times more likely to develop the disorder, while first-degree relatives of those with MA are approximately four times more likely to develop it [23]. Twin and family studies from the 1990 s revealed significant hereditary factors, with heritability estimates ranging from 35 to 60% [24, 25]. Patients diagnosed with migraine as their initial diagnosis at a younger age typically exhibit a greater genetic predisposition to migraine than the overall migraine population [26]. A recent British study [26] identified increased heritability based on single nucleotide polymorphisms (SNPs), highlighting risk loci linked to genes such as PRDM16, FHL5, ASTN2, STAT6/LRP1, and SLC24 A3 in individuals experiencing MO or MA as their first diagnosis.
A recently published review article [27] thoroughly describes HM, gene mutations, and other monogenic variants of migraine, and therefore, these topics will not be addressed in this systematic review.
Genome-Wide Association Studies (GWAS) in MA
Although numerous GWAS studies have been conducted, few specifically focus on MA. Two studies [28, 29] identified genetic differences between MA and MO. The 2010 study highlighted the sequence variant rs1835740 (chromosome 8q22.1) as a significant risk factor for MA, while a 2013 meta-analysis of 29 GWAS studies found no genome-wide significant loci unique to MA, whereas MO had six associated loci.
A large-scale GWAS [30] confirmed that seven loci were significantly associated with MO (associated with the genes encoding TSPAN2, TRPM8, PHACTR1, FHL5, ASTN2, FGF6, LRP1). However, none were exclusive to MA, likely due to the smaller sample size and greater clinical heterogeneity. A more recent GWAS [31] analysed 102,084 migraine cases and 771,257 controls, identifying three loci specific to MA (associated with the genes HMOX2, CACNA1 A, MPPED2), reinforcing the distinct genetic profile and neurovascular involvement of MA.
Polygenic and gene-based approaches in MA and MO
A Finnish study [32] analysed polygenic risk scores in 8,319 individuals from 1,589 families affected by migraine. It demonstrated that MA had a higher genetic risk than MO, with polygenic risk scores accounting for 2.9% of the variance in MO, 5.5% in MA, and 8.2% in HM. Familial cases of MA exhibited higher polygenic risk scores than population-based cases, and some were associated with rare Mendelian mutations (in the genes CACNA1 A, ATP1 A2, SCN1 A), particularly in familial hemiplegic migraine.
A gene-based pleiotropy analysis [33] examined 4,505 MA cases vs. 34,813 controls and 4,038 MO cases vs. 40,294 controls, identifying 107 shared genes. Six genes (TRPM8, UFL1, FHL5, LRP1, TARBP2, NPFF) were significantly associated with both MA and MO, supporting a shared genetic basis with some subtype-specific variations. A genetic risk score study [34] analysed previously identified SNPs and found that common migraine variants were more predictive of MO than MA, suggesting that rare variants rather than common SNPs may influence MA.
Pathophysiology
The pathophysiology of migraine aura is most commonly explained by cortical spreading depression (CSD). CSD is characterized by a slowly spreading wave of cortical neuronal and glial cell depolarization, followed by a depression of electrical activity [35]. This process was initially reported by Leão in 1944, and its involvement in migraine aura was later confirmed through functional neuroimaging and blood flow investigations [36]. CSD spreads at a rate of 3 to 5 mm/min, leading to significant changes in transmembrane ion gradients. This results in a large influx of water, Na+, and Ca2+, accompanied by an efflux of K+, protons, glutamate, and ATP [35]. The localised increase in extracellular potassium (K+) disrupts normal ionic gradients, depolarising adjacent neurons and glia, ultimately causing neuronal swelling. The subsequent release of glutamate stimulates nitric oxide production, resulting in vasodilation and a transient increase in local blood flow [36]. The activation of the trigeminovascular system, crucial to the pathophysiology of migraine, may be triggered by the release of pro-inflammatory and excitatory mediators, including nitric oxide, glutamate, and adenosine triphosphate, during CSD transmission. These mediators stimulate meningeal and perivascular trigeminal nociceptors, resulting in migraine pain [36].
Furthermore, the thalamus and hypothalamus are recognised as significant contributors to migraine aetiology, alongside cortical involvement. Both the premonitory and aura phases of migraine exhibit altered hypothalamic connections with the trigeminal system and brainstem nuclei [35]. CSD is also linked to vascular changes throughout a migraine attack. As the depolarization wave recedes, a period of oligemia follows the initial hyperemia [36]. This one-to-two-hour hypoperfusion phase is thought to exacerbate migraine symptoms by demonstrating the complex interactions among inflammatory, vascular, and neuronal processes related to migraine aura [35, 36].
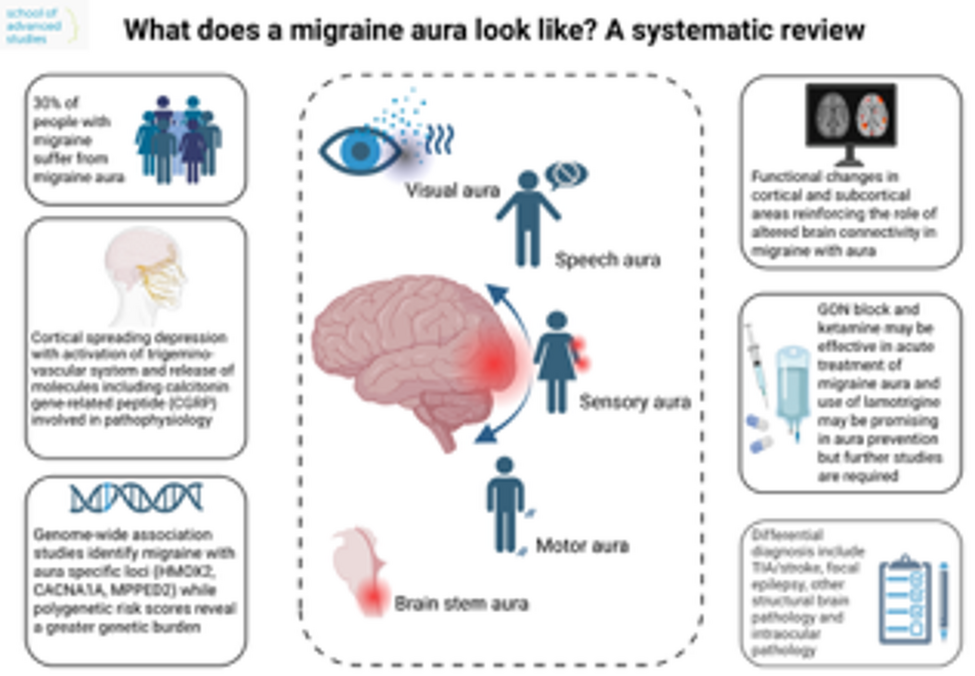
Different aspects of migraine auraVisual aura
The visual symptoms described in the visual aura are heterogeneous, and currently, there is no consensus on the terminology for elementary visual symptoms during visual aura. Descriptions of visual aura symptoms have primarily been based on either fixed response questionnaires or health professionals’interpretations of patients’verbally reported experiences of visual aura symptoms. Recent studies [7, 8] have sought to compile a comprehensive list of visual aura symptoms reported by migraine patients.
The available evidence regarding two major features of visual aura, its duration and characteristics, is summarised in Tables 1 and 2. Five studies, two of which involved the same population, reported the duration of visual aura using various time intervals. The most common durations were 5–30 min (72%) [37], 5–60 min (92%) [38], 5–30 min (65.5%) [39], 21–30 min (37%) [6], and 5–60 min (86%) [9]. All five studies indicated that visual aura could last more than 60 min: 10% [37], 8% [40], 6.6% [39], 14% [6], and 10.2% [9]. In ten studies, the characteristics of visual aura were identified (Table 1). Most patients were recruited from neurology or headache centers at university hospitals (women 63%−90.3%, mean age 30–46). Data collection on visual aura characteristics was primarily based on retrospective recall, utilising questionnaires or semi-structured interviews. Three studies collected visual aura characteristics prospectively [8, 40, 41]. The number of different types of visual aura symptoms varied, ranging from three [41] to 26 [7]. The most common visual aura characteristics identified in each study included scintillating scotomas and fortification spectra (38%) [41], flickering light (326/358 = 91%) [37], scintillating scotoma (a propagating “crescent” of the homonymous type) (112/178 = 62%) [42], ‘foggy’or blurred vision (66/122 = 54.1%) [39], dots or flashing lights (188/267 = 70%) [43], flashes of bright light (25/72 = 35%) [8], scintillating scotoma (22/23 = 96%) [40], flickering or bright light (25/55 = 46%) [7], and gradually spreading positive symptoms (192/215 = 89.3%) [9]. Only one prospective study [8] recorded participants’ descriptions of visual aura symptoms in a free text diary, allowing further categorisation of visual disturbances into 20 elementary visual symptoms. In a recent study [7], visual aura was systematically subdivided into 25 elementary visual symptoms (images representing 25 elementary visual symptoms) to create a standard migraine aura iconography (SMAI) with accompanying text descriptions. The 25 elementary visual symptoms include: bright light, ‘foggy’ or blurred vision, zig-zag lines, single scotoma, multiple scotomas, small bright dots, white dots or round forms, colored dots or round forms, colored lines, prisms or geometrical shapes, the sensation of looking through heat waves, water or oil, tiny flickering dots, ‘bean-like’ forms, hemianopsia, deformed images, tunnel vision, oscillopsia (movement of stationary objects), mosaic vision, fractured objects, corona effect (extra edge on objects), total blindness, micropsia, macropsia, altered colors, and complex hallucinations. The SMAI was tested through a web-based survey (smartphone or computer) among participants with migraine with aura. Participants were instructed to recall all the various elementary visual symptoms they had previously experienced, and then they were presented with individual images of these symptoms one at a time. If recognised, a new elementary visual symptom image would be presented. If not recognised, a written description would be shown, and if recognised via text, the participant would be prompted to describe the elementary visual symptom in free text for feedback. The majority of participants (98%) were able to identify at least one image from the first version of SMAI (version 1.0) as representing a visual disturbance they had experienced. In total, participants recognised 78.4% of the elementary visual symptoms as part of their visual aura based solely on the standardised iconography images. Based on the results, the authors produced the amended SMAI 2.0 to reflect a simpler visual scenery (two air balloons) with superimposed elementary visual symptoms and included a newly reported elementary visual symptom (curtain phenomenon), resulting in a 26-element visual aura iconography tool available online [7].
Table 1 Studies reporting duration of visual auraTable 2 Summary of studies reporting visual aura characteristicsSensory and speech and/or language aura
According to ICHD-3 [4], aura with sensory symptoms (SA) and aura with speech and/or language disturbances (SLA) are categorised as typical aura [4]. These auras are less common than visual auras but are certainly not rare– approximately 1 in 3 and 1 in 7 migraineurs with aura experience SA and SLA, respectively [9]. SA is commonly characterised by positive symptoms such as paresthesias (often described as”pins and needles”) in the face, arm, and/or hand, which gradually spread from the initial site to adjacent areas. Therefore, in cases of sudden-onset negative symptoms (hypesthesia, anaesthesia), thorough consideration should be given to differential diagnoses and the possibility of secondary aetiologies, even though these symptoms may also be manifestations of SA. Notably, approximately two-thirds of SAs present as unilateral conditions, with half of the patients exhibiting side-locked symptoms [9]. SLA is inherently a unilateral symptom [4]. It typically manifests as non-fluent aphasia, where patients encounter challenges with speech production (e.g., paraphasia or anomic aphasia). Dysarthria affects about one-third of patients with SLA [8, 9]. SLA usually occurs alongside visual aura or SA. Isolated SLA is uncommon, and its etiology deserves thorough investigation [9].
Approximately one-quarter of patients experience typical aura symptoms simultaneously, but these symptoms are more likely to occur sequentially [6, 9]. The second symptom may arise while the first symptom is ongoing, at the end of the first symptom, or after a symptom-free interval. It is commonly believed that the first symptom of migraine aura is visual, which then progresses to sensory and eventually to speech disturbances, reflecting the presumed anterior propagation of CSD. This description aligns with the most common clinical scenario; however, the initial aura symptom can be any of the aforementioned types [9]. The median duration of SA and SLA is 20 min; however, auras lasting more than 60 min are not infrequently encountered, comprising 21% and 6% of recorded SA and SLA, respectively, which may lead to diagnostic confusion [6]. Prolonged auras (lasting more than 60 min) have a statistically significant higher incidence of SA and SLA– 68% and 31%, respectively– which may be pathophysiologically linked to the fact that cortical spreading depression has more time to spread to adjacent brain areas [45]. Furthermore, recent studies have documented a decreasing prevalence of SA, SLA, and prolonged aura with advancing patient age [46, 47].
Motor aura
HM, a rare subtype of MA, is characterised by temporary motor weakness, typically lasting no more than 72 h [4]. It can present as either sporadic hemiplegic migraine, which occurs without a family history, or FHM, which exhibits an autosomal dominant inheritance pattern [48]. The prevalence of HM in the general population is estimated to be about 0.01%, indicating a low overall incidence [48]. An essential feature of hemiplegic migraine is motor aura, which is present in all cases, although its intensity and duration can vary widely between individuals [49, 50]. Comprehensive recognition of the clinical symptoms associated with motor aura is crucial for effective diagnosis, as these symptoms can mimic those of cerebrovascular events [50]. Motor aura often begins as unilateral weakness that progressively worsens over at least five minutes, affecting the ipsilateral face and/or limbs [49]. Other aura symptoms, including hemianopsia, scintillating scotomas, visual field defects, ataxia, fatigue, and sensory disturbances, are commonly observed in HM alongside motor weakness [50]. The aura phase typically lasts between 20 and 60 min; hemiplegia and altered consciousness can persist for weeks before complete recovery from severe migraine attacks, though full recovery from these attacks is common [48]. Headache is nearly always experienced during an episode, and it is frequently quite severe. Headache laterality has been reported as ipsilateral, contralateral or bilateral to the motor symptoms [48, 50]. Notably, HM can also be associated with seizures, especially in some individuals with familial hemiplegic migraine type 2 (FHM2), which is caused by ATP1 A2 gene mutations [50, 51].
Retinal migraine
Retinal migraine, although included in ICHD-3 [4], remains a controversial entity. It must fulfil the criteria for MA. The aura must be fully reversible, monocular, and present as positive and/or negative visual phenomena (e.g., scintillations, scotoma, or blindness). This confirmation must occur during an attack, either through a clinical visual field examination or by having the patient draw a monocular field defect after clear instructions. Additionally, the aura should exhibit at least two of the following features: gradual spreading over at least 5 min, symptoms lasting 5 to 60 min, or being accompanied or followed within 60 min by a headache. Visual symptoms that meet ICHD-3 criteria for retinal migraine are incredibly rare, and previous reviews have only identified a limited number of case reports of transient monocular vision loss believed to represent retinal migraine [52, 53]. Our literature search did not uncover any prospective or retrospective studies on retinal migraine but did reveal two case reports of altered monocular retinal perfusion documented by fundus photography/videography and fluorescein angiography [54, 55]. Arguably, neither case fully satisfied the ICHD-3 criteria for retinal migraine.
Migraine with brainstem aura
Migraine with brainstem aura (MBA), formerly known as basilar migraine, is a rare subtype of migraine [56]. To diagnose MBA, at least two of the following symptoms must be present: dysarthria, vestibular disturbances, tinnitus, hyperacusis, diplopia, ataxia not due to sensory deficits, or a transiently decreased level of consciousness (GCS ≤ 13), without motor or retinal symptoms [4]. The aura typically lasts between 5 and 60 min; however, in rare cases, it may persist for up to 72 h. It often precedes the headache in 87% of cases or occurs simultaneously in 13% of cases [57]. Yamani et al. [58] reviewed 79 reported cases of MBA and found that 56% met the ICHD-3 criteria. Among 293 patients diagnosed with migraine aura at the Danish Headache Center, 1.37% were diagnosed with MBA, representing 0.04% of the general population. A follow-up survey identified MBA in 12.8% of 1781 patients with aura. Ying et al. [59] conducted a cross-sectional study of patients presenting with headaches at a university hospital outpatient neurology clinic, identifying MBA in 1.5% (23 out of 1526) of evaluated patients. The average age of onset was 20 years (range 6–49), with more than half experiencing their first attack in childhood or adolescence. In two-thirds of the patients, the headache was bilateral, pulsating, moderate to severe in intensity, and resolved within 24 h. Among the symptoms of MBA, diplopia was the most common (52%), followed by vertigo and tinnitus (both 43%), bilateral visual disturbances (39%), hypoacusis and ataxia (26% each), dysarthria (22%), and bilateral paresthesias and transient loss of consciousness (both 13%). Rare manifestations of MBA may include complications such as coma or prolonged confusion, which are more frequently observed in children and young adolescents. Severe cases have been documented, with a literature review identifying 23 reported instances of MBA associated with coma [60]. Disturbed consciousness typically lasts for a few seconds to several minutes and often resolves within 30 min [61]. Clinically significant EEG slowing or generalized spike-and-wave complexes, which may persist for several days, can be observed in adult and adolescent patients with impaired consciousness and brainstem aura [62]. The underlying mechanisms remain unclear, but in most patients, MBA symptoms tend to evolve over the course of life into more typical manifestations of migraine with aura or resolve entirely [63].
Neuroimaging
Neuroimaging serves two distinct purposes in the context of migraine aura: it may assist in the clinical assessment of individual patients [64], and it has significantly contributed to the understanding of underlying pathophysiological mechanisms through group-level research [65]. The following sections first address indications for diagnostic imaging in clinical practice, followed by an overview of structural and functional neuroimaging findings from research studies involving patients with migraine aura (MA).
Diagnostic neuroimaging in clinical practice
Diagnostic neuroimaging, primarily computed tomography (CT) scans and magnetic resonance imaging (MRI), is generally not recommended. However, it should be conducted in patients presenting with atypical symptoms, such as prolonged aura or concerning details in their medical history, to evaluate possible causes of secondary headache [66, 67].
Structural MRI
In migraine auras, white matter hyperintensities (WMH), characterised by an increased signal intensity in T2-weighted and Fluid-Attenuated Inversion Recovery MRI (FLAIR) sequences [68], are commonly observed and occur significantly more frequently than in healthy controls [69, 70]. The incidence appears to increase with age [71], particularly among women with MA [72]. Other factors that elevate the likelihood of WMH occurrence in migraine sufferers include persistent foramen ovale (PFO) [73] and thrombophilia [74]. Conversely, two studies examining the influence of C-reactive protein and right-to-left shunts have found no significant correlation with the occurrence of WMH, resulting in conflicting reports on this topic. Some studies indicate no significantly higher occurrence of WMH in patients with MA compared to healthy controls [75,76,77]. However, longitudinal analyses suggest a progressive increase in WMH burden over time and a correlation between the duration of aura and the number of WMH [78]. Nonetheless, other symptom modalities (e. g., frequency, severity) have not been shown to influence the occurrence of structural changes [79, 80]. Changes in cortical structure have been observed in migraine patients compared to healthy controls, although findings vary across studies. Decreases in grey matter volume have been noted in the occipital cortex and other visual processing regions [81], the left inferior temporal lobe, and the right cerebellum [82]. Studies on cortical thickness have shown inconsistent results, with some reports indicating increased cortical thickness in visual processing areas [79, 83], while others found no differences in cortical structure between MA patients and healthy controls [84]. These discrepancies may reflect methodological differences in image processing, analysis, and statistical approaches, along with variability within patient populations. Subcortical structures such as the hippocampus [40], thalamus [85], and brainstem [86] have also exhibited volume changes. Occipital bending, which describes an asymmetry of the occipital lobes where one lobe extends over the midline, is reported to occur significantly more frequently in MA than in migraineurs without aura [87]. Diffusion tensor imaging parameters did not differ between MA patients and healthy controls, suggesting no variations in white matter microstructure [88].
Functional neuroimaging findings
Functional neuroimaging, particularly resting-state fMRI, enables non-invasive, real-time observation of brain activity. It can detect changes in brain activity during the aura, aiding in the identification of brain regions involved in the onset and progression of aura, as well as network connectivity. The blood oxygenation level-dependent (BOLD) signal measures brain activity used in fMRI, detecting variations in blood oxygen levels that occur when neurons are active [89]. Intra- and interictal resting-state functional connectivity increases have been identified between the left pons and the left somatosensory cortex, between visual cortex area V5 and the frontal cortex [90], in the right lingual gyrus [91], and within the default mode network [92]. Conversely, reduced connectivity has been observed between the anterior insula and occipital areas [92], suggesting a disruption between the salience and visual networks. Furthermore, hyperexcitability in specific non-occipital cortical areas, such as the inferior frontal gyrus, superior parietal lobule, and intraparietal sulcus, has been noted, often occurring contralateral to visual aura symptoms [93]. BOLD-signal variability has been shown to correspond with and vary based on aura symptoms. Notably, patients who experience only positive symptoms, such as flickering lines or spots, exhibit an increased BOLD response [94]. These BOLD fluctuations are more pronounced in patients with MA [95]. Hypoxia serves as a modulating factor for BOLD signals, with MA patients showing a more significant decrease in BOLD response [96].
Functional neuroimaging techniques, such as positron emission tomography (PET), magnetic resonance spectroscopy (MRS), and magnetoencephalography (MEG), have provided further insights. Reduced levels of choline in the cerebellum have been noted in patients with MA, potentially indicating disruptions in cell membrane metabolism [97]. Conversely, GABA levels in the occipital and somatosensory cortex have not demonstrated significant differences between MA patients and healthy controls [98].
Electrophysiology
Electrophysiological techniques have been instrumental in advancing our understanding of migraine with aura (MA). Non-invasive methods such as electroencephalography (EEG), visual evoked potentials (VEP) and transcranial magnetic stimulation (TMS) have revealed distinct abnormalities in cortical excitability, sensory processing, and neural connectivity in MA patients [62, 99].
Electroencephalography (EEG)
EEG has been used in the clinical study of migraine since as early as 1947 [100]. In recent years, quantitative EEG (qEEG) has become central to migraine research, using spectral and coherence analyses to examine neural oscillations and connectivity [101]. The most consistent interictal EEG finding is a diffuse slowing of brain activity, with decreased alpha and beta power and increased theta and delta rhythms [102]. In MA, this pattern includes reduced beta activity and increased alpha power in regions affected by the aura [103], along with a pronounced increase in theta-band activity [104]. Some studies have even found that EEG can distinguish between MA and MO based on theta activity levels [105]. A promising application of EEG lies in the detection of cortical spreading depression, which has been observed using invasive recordings and new analytical methods [106,107,108,109].
Visual evoked potentials (VEP)
Patterns of cortical excitability have been studied using visual evoked potentials in MA patients. The majority of studies analysed exclusively visual aura, probably due to its higher prevalence [62]. There is conflicting available data on VEP parameters interictally, with some studies showing increased interictal VEP amplitudes (reflecting stronger visual cortex responses to visual stimuli) in MA patients when compared to controls or even MO patients [110,111,112,113,114,115]. Another study found decreased VEP amplitude values in MA patients [116]. Similarly, there have been findings of increased interhemispheric response asymmetry, related or not to the side of the visual aura, that were inconsistent in subsequent studies [111, 117].
A recent study has identified distinct interictal VEP patterns in MA patients with different aura phenotypes, including purely visual auras and more complex auras with paraesthesia and/or dysphasia, when compared to healthy controls [110]. In general, MA patients showed reduced VEP N1-P1 habituation, which may reflect impaired sensory adaptation and cortical inhibitory mechanisms. The authors advocated that different aura phenotypes may be explained by different neurophysiological responses, which in turn should be an area of further research [110]. During the visual aura, a suppression or complete abolition of the first three components of the flash VEPs has been demonstrated within the hemisphere contralateral to the aura, with normalisation of all impaired neurophysiological values during the following headache phase [118, 119]. In persistent aura without infarction a more pronounced P100 response to checkerboard activation has been found when compared with MA and MO patients [120]. An important limitation is that none of the studies recorded VEP according to ISCEV (International Society for Clinical Electrophysiology of Vision) standards for visual evoked potentials [121].
Transcranial magnetic stimulation (TMS)
Transcranial magnetic stimulation is a non-invasive method used to assess and modulate cortical excitability, which has been widely applied in migraine research since the 1980 s [122]. In migraine with aura, TMS studies have revealed abnormal excitability and plasticity in both the motor and visual cortices. Phosphene thresholds (PTs), used to measure visual cortex responsiveness, are often lower in MA, indicating increased cortical excitability, especially in response to excitatory stimuli [123,124,125,126]. MA patients also exhibit impaired synaptic plasticity, particularly a failure of long-term depression mechanisms. They respond less to inhibitory TMS protocols compared to those without aura and healthy controls, suggesting a reduced capacity for cortical inhibition [127,128,129,130,131,132].
Differential diagnosis
The differential diagnosis of MA includes conditions that present with transient neurological symptoms, such as transient ischemic attack (TIA), stroke, focal epileptic seizures, structural brain pathology, and ophthalmological or psychiatric disorders [133]. Diagnosing MA is particularly challenging in cases of aura without headache, atypical aura, and among older patients, as well as those with cardiovascular risk factors, a previous history of epilepsy, or comorbid ophthalmological or psychiatric disorders. Additionally, secondary headache causes, such as headaches following an acute stroke or post-ictal headache, may exhibit migrainous features [35]. An adequate differential diagnosis of transient neurological deficits is crucial to avoid unnecessary treatment approaches, including intravenous thrombolytics or high doses of antiseizure drugs. Table 3 summarizes various conditions that mimic migraine aura [4, 36, 133,134,135,136,137].
Table 3 Differential diagnosis of migraine auraTIA/stroke
MA accounts for approximately 9% of stroke mimics [138, 139]. The clinical symptoms of TIA/stroke often overlap with those of migraine aura, complicating accurate diagnosis, particularly in patients with risk factors for both conditions [140]. Clinically, cerebral ischemia usually manifests with a sudden onset of focal neurological deficits that do not progress over time, typically including multiple symptoms that occur simultaneously. These symptoms are generally negative, such as hemianopia, hemiparesis, or hemianesthesia [36, 133]. The ICHD-3 indicates that headaches may accompany ischemic strokes and can display migrainous characteristics [138]. In most instances, neuroimaging does not show specific findings during a migraine aura; however, it may occasionally assist in differentiating it from an acute ischemic event. During the aura phase, studies utilizing perfusion-computed tomography (PCT) and perfusion-MRI have revealed hypoperfusion patterns that are not limited to a single vascular territory [139, 141, 142]. In contrast, acute stroke imaging on MRI typically indicates diffusion restriction and hypoperfusion confined to one vascular territory [141, 142].
Focal epilepsy
A recent review by Paungarttner et al. highlights the commonalities and differences between epilepsy and migraine [143]. Epilepsy, particularly focal epileptic seizures, can present focal neurological symptoms that resemble the aura of a migraine. This phenomenon is especially frequent in occipital lobe epilepsy, which may cause transient visual symptoms that are often stereotyped and repetitive, typically consisting of elementary hallucinations such as coloured shapes or spots [36]. A small retrospective study suggests that the duration of an epileptic visual aura is shorter (ranging from a few seconds to a few minutes), has a higher likelihood of being stereotyped, is more likely to be confined to one visual hemifield, and does not display centripetal or centrifugal progression of visual symptoms, in contrast to migraine [35, 134]. Headache may coexist in patients with focal seizures and during the postictal phase, usually occurring ipsilaterally to the seizure activity, as classified in the ICHD-3 [4]. If focal epileptic seizures are suspected, MRI can assist in identifying structural lesions, and electroencephalograms may reveal focal cortical epileptic activity [36].
Structural brain pathology
Several structural brain lesions can produce symptoms similar to those of migraine [133]. Cerebral amyloid angiopathy (CAA) may mimic migraine aura, particularly in cases of late-onset (after 50 years of age). Transient focal neurological deficits are well-documented symptoms of CAA, typically comprising progressive, short-lived positive symptoms known as “amyloid spells”, which may include visual disturbances such as blurred vision and flickering lights, along with somatosensory symptoms like unilateral paresthesia. Headache may accompany these transient neurological events, especially in the context of new intraparenchymal bleeding. Although the mechanisms behind these symptoms remain unclear, they may involve cortical spreading depression caused by amyloid and hemosiderin deposition in the cerebral cortex. Blood-sensitive MRI sequences may help identify amyloid deposits and cerebral microbleeds in these patients. Other structural brain conditions that may mimic migraine aura are described in the literature, including brain tumors [144], posterior reversible encephalopathy syndrome (PRES) [145], subarachnoid haemorrhage [146], arteriovenous malformation [147, 148], dural arteriovenous fistula [149], posterior circulation embolism [150], internal carotid artery dissection [151], vertebral artery dissection [152], carotid artery stenosis [153], and Moyamoya disease [154].
Other rare conditions, including CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy), atrial myxoma, autoimmune disorders (such as cerebral vasculitis, systemic lupus erythematosus, antiphospholipid antibody syndrome, and Sneddon’s syndrome), haematological disorders (such as essential thrombocythemia, polycythemia vera, and hereditary hemorrhagic telangiectasia), leptomeningeal angiomatosis (Sturge-Weber syndrome), and Hashimoto’s encephalopathy, can also mimic migraine aura [133, 155].
Ophthalmological disorders
Acute angle closure is an ophthalmological emergency that can mimic a migraine with visual aura. It predominantly affects the elderly and is characterised by sudden monocular vision loss, often presenting as blurred vision and/or rainbow-coloured halos, accompanied by headache or acute eye pain, frequently associated with nausea and vomiting. Clinical examination typically reveals a combination of a poorly reactive mid-dilated pupil, ciliary injection, corneal oedema, and increased intraocular pressure [136].
Posterior vitreous detachment manifests as sudden, painless flashes or floaters in one eye. It may lead to a retinal tear that, if left untreated, can progress to retinal detachment. The primary symptom of retinal detachment is loss of visual field in one eye [145]. Optic neuritis typically presents with subacute, painful vision loss, dyschromatopsia, and increased pain during eye movements [145].
Acute retinal ischemia (including cilioartery occlusion, branch retinal artery occlusion, and central retinal artery occlusion) presents as sudden, painless central vision loss or total blindness. It is often linked to other vascular risk factors [145].
Other disorders
Visual snow syndrome (VSS) is a neurological condition characterised by visual symptoms such as dynamic, continuous, tiny dots resembling television static, palinopsia (trailing of moving objects or after images), enhanced entoptic phenomena (such as excessive floaters or spontaneous photopsia), photophobia, and nyctalopia (impaired night vision), persisting for over three months [4]. VSS appears to be epidemiologically linked to migraines. The pathophysiology is not fully understood but may relate to dysfunctional sensory processing and excitability of the occipital cortex [143, 156]. Reports indicate that improvements may occur with lamotrigine and topiramate [157, 158]. Mindfulness-based cognitive therapy has shown effectiveness in alleviating VSS symptoms and modifying functional connectivity in visual networks [159].
Charles Bonnet Syndrome is a benign clinical condition characterized by visual hallucinations—either simple or complex, such as faces, animals, or objects—that occur in patients with established significant vision loss, most often resulting from macular degeneration, diabetic retinopathy, glaucoma, optic atrophy, or retrogeniculate visual pathway pathology [36, 137].
Treatment
Most studies report the effectiveness of abortive or preventive medications for pain relief, but they do not address their effects on the duration, severity, or frequency of aura. However, aura can be a troubling symptom, whether due to its high frequency or duration (exceeding 60 min), or the level of anxiety or disability it may cause, such as severe language disturbances, hemiparesis, or significant brainstem symptoms. Currently, no well-established treatments exist to abort or prevent aura symptoms [160, 161].
Acute treatment of migraine aura
Several case reports have utilized greater occipital nerve (GON) blockade to rapidly and safely terminate prolonged auras. While the exact mechanism remains unclear, it is hypothesised that the GON blockade modulates the activity of the trigeminocervical complex, potentially aborting the CSD [161,162,163]. A prospective, open, non-controlled pilot study using bilateral GON blocks (2 ml of 0.5% bupivacaine) to treat prolonged aura (lasting between two hours and one week) demonstrated efficacy in approximately 85% of cases (22 episodes). A complete response without recurrence was achieved in 11 out of 22 (50%) aura episodes, while a complete response with recurrence within 24 h occurred in 2 out of 22 cases (9.1%). Additionally, a partial response with 50% improvement was noted in 6 out of 22 cases (27.3%). The maximum response was recorded at a median of 10 min (interquartile range 6–30 min). The GON blockade proved to be more effective in patients experiencing less frequent and shorter-lasting auras. Although the results appear promising, the sample size and study design (which did not control for the placebo effect) may limit their interpretation [164].
A double-blind, randomised parallel-group controlled study using intranasal ketamine demonstrated a reduction in the severity of prolonged migraine aura, although it did not affect its duration. While this well-designed study (utilising midazolam as a control, and excluding patients taking triptans or ergotamine, as well as those experiencing recent changes in prophylactic treatment) has merit, the small sample size and limited number of aura episodes per patient represent significant limitations [165]. A previous small open-label study reported a reduction in both the severity and duration of aura; however, it’s even smaller sample (comprising only FHM patients) and the absence of masked randomisation and a control group present critical shortcomings [166]. As an NMDA glutamate receptor antagonist, ketamine has been found to block CSD in animal studies, and the authors hypothesise that this is the mechanism underlying its effect on aura [165].
There is anecdotal evidence supporting the benefits of intravenous verapamil [167], intravenous furosemide [168], intravenous prochlorperazine combined with magnesium [169], and acetazolamide [170, 171]; however, this does not provide consistent proof of efficacy. In a well-designed single-centre pilot study, a combination of Tanacethum Parthenium, 5-hydroxytryptophan, and magnesium was found to shorten the aura, reducing disability in 96% (48/50) of patients, but these findings require further validation in larger controlled trials [172]. Most studies do not address the effect of triptans on the aura itself. A double-blind placebo-controlled study concluded that eletriptan did not influence aura duration (neither shortening nor prolonging) when administered during the aura phase; however, this study was not primarily designed to evaluate the effect on aura [173].
Preventive treatment of aura
There are conflicting results regarding the benefits of topiramate. A prospective open-label study did not demonstrate the effectiveness of topiramate in reducing the frequency and duration of migraine aura in 12 patients. The very small sample size, the uncontrolled nature of the study, and the selection bias limit the interpretation and generalisation of the data [174]. In contrast, a 12-month post hoc analysis of the Prolonged Migraine Prevention with Topiramate (PROMPT) trial showed a significant reduction in the number of migraine auras, along with a decrease in headaches, in a large sample (despite a 32% drop-out rate) [175].
Lamotrigine works by blocking voltage-sensitive sodium channels and inhibiting the release of glutamate from neurons, which is thought to be involved in CSD [160, 176]. Pascual et al. [176] reported a reduction of over 50% in auras for 68% (30/44) of patients (from 4.2 to 0.7 episodes). In 9 out of 13 patients who discontinued lamotrigine after 6 to 12 months, there was a prompt reappearance of auras. This was an open trial with a relatively small sample (including only patients with intense and frequent auras) and a partially retrospective nature; therefore, these results should be interpreted with caution. A controlled prospective open trial further confirmed the specific benefit of lamotrigine regarding aura: lamotrigine reduced the frequency (from 1.5 (SD 0.6) to 0.4 (SD 0.7) episodes per month) and the duration of aura (from 26.9 (SD 10.8) to 8.3 (SD 13.6) minutes), irrespective of aura type. The authors also found a significant correlation between the reduction in the frequency of migraine aura and headache episodes. As in the previous study, the absence of a placebo group and the sample size limit the generalisation of the results [177]. In a 5-year follow-up report of three cases of migraine with brainstem aura treated with lamotrigine at a dosage of 100 mg/day, remission of episodes was achieved, with the reappearance of aura noted when the patients discontinued lamotrigine. While this small case series provided an extended follow-up period, further trials with larger samples are needed to confirm these results [178].
In a retrospective case series involving 11 patients with hemiplegic migraine, an injection of onabotulinumtoxinA (administered using a modified version of the PHASE III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) protocol) reduced the frequency, severity, and/or duration of their sensorimotor aura. The authors hypothesise that onabotulinumtoxinA lowers calcitonin gene-related peptide (CGRP) levels, thereby interfering with CSD [179]. The retrospective nature and small size of the cohort necessitate further validation in larger trials.
Concerning monoclonal antibodies targeting the CGRP pathway, Ashina et al. performed a post hoc secondary analysis of four double-blind, placebo-controlled randomised clinical trials using erenumab (70 mg or 140 mg once monthly) for MA and MO. The authors did not observe a statistically significant reduction in monthly aura days [161]. However, this analysis was not specifically intended to address aura; only a subset of patients with chronic migraine aura was evaluated, which limits the generalisability of the results and highlights the need for further trials to investigate the effect of CGRP blockade on migraine aura.
A randomised, double-blind, placebo-controlled crossover trial involving tonabersat (a neuronal gap-junction blocker that inhibits CSD in animal models) reported a significant reduction in the number of aura attacks. While this trial was well-designed, the data require confirmation in larger cohorts [180]. A single prospective observational pilot study found that the use of estrogen-free desogestrel (75 mcg/day)-containing oral contraception significantly reduced the duration of aura (p 181]. Valproic acid has been reported as useful in three patients with persistent migraine aura due to its GABAergic activity, which could interfere with CSD [182, 183]. There are anecdotal reports of success in reducing the duration and frequency of the migrainous aura with ginkgo biloba [184] and picotamide [185]. However, these reports were not followed by further studies to confirm their findings.