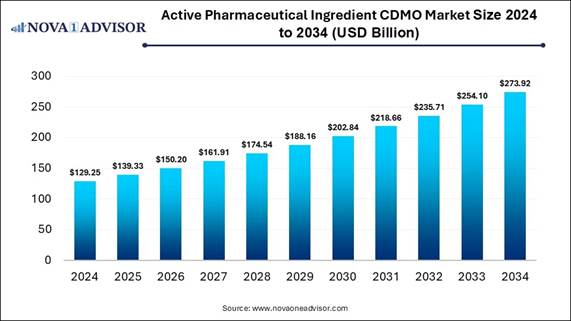
According to Nova One Advisor, the global active
pharmaceutical ingredients CDMO market size is expected to be
worth around 273.92 billion by 2034, increasing from USD 139.33 billion in 2025,
representing a healthy CAGR of 7.8% from 2025 to 2034.
The active pharmaceutical ingredients CDMO
market is expanding as partnering with CDMOs by pharmaceutical organizations increases
their ability to solve some of the most persistent challenges faced by drug
manufacturers. This partnership enables a drug developer to avoid the massive
capital expenditure needed to equip, build, and staff its own cGMP-compliant
manufacturing services.
• A CDMO offers enthusiastic subject matter
experts during the development and manufacturing process who have a wealth of
experience, knowledge, and can tackle probable challenges.
The Complete Study is Now Available for
Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/8371
Active Pharmaceutical Ingredients CDMO
Market Highlights:
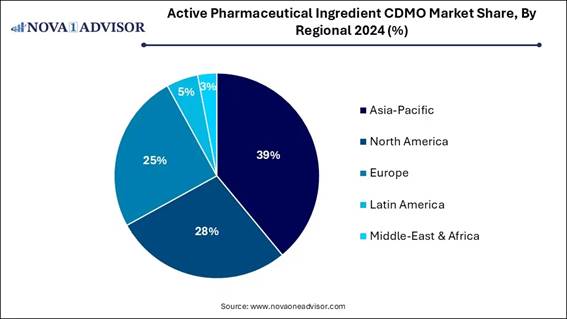
🔹Asia Pacific dominated the overall Active Pharmaceutical Ingredients
CDMO market with a revenue share of 39% in 2024.
🔹The U.S. accounted for a dominant revenue share in the API CDMO
market in North America in 2024.
🔹The traditional active pharmaceutical ingredients segment held the
largest share of 40% in 2024.
🔹The antibody-drug conjugate segment is expected to show lucrative
growth during the forecast period.
🔹The synthetic segment dominated the API CDMO market and accounted
for the largest revenue share of 74% in 2024.
🔹The biotech segment is estimated to register a faster CAGR over the
forecast period.
🔹The innovative drugs segment dominated the API CDMO market and
accounted for the largest revenue share of 74% in 2024.
🔹The generic segment is expected to showcase lucrative growth over
the forecast period.
🔹The oncology segment dominated the active pharmaceutical ingredients
CDMO market and accounted for the largest revenue share of 36% in 2024.
🔹The glaucoma segment is expected to experience lucrative growth over
the forecast period.
🔹The commercial segment held a dominant market share of 91% in 2024.
🔹The clinical segment is expected to showcase lucrative growth over
the forecast period.
Market Overview and Industry Potential
CDMOs provide a wide range of solutions in
the pharmaceutical
development and manufacturing techniques, including drug substance such as
active ingredients manufacturing, drug product manufacturing, analytical
testing and quality control techniques, process development and
optimisation, technology transfer, scale-up from clinical to commercial
manufacturing, government compliance support, and packaging and distribution.
It also provides a combination of both research and manufacturing solutions.
⬥︎ For Instance, In April 2025, Sun
Nuclear, a Mirion Medical company, announced the acquisition of Oncospace, a
company offering cloud-based, AI-driven solutions for the radiation oncology
community. This strategic move underscores the Sun Nuclear commitment to
leveraging cutting-edge technology to improve treatment outcomes on behalf of
customers.
Recently, CDMOs have adopted sustainable
practices such as using single-use
bioreactors, continuous flow manufacturing, and biocatalysis to cut
waste and boost efficiency. Those that actively incorporate sustainability into
their corporate strategy are more likely to attract customers and build lasting
partnerships. The main advantage of this technology is its ability to lower
energy use, thanks to more efficient heat and mass transfer. As a result, drug API
companies are increasingly choosing CDMOs committed to environmentally friendly
operations.
Latest Trends of the Market
⬥︎ In July 2025, Phlow Corp., a leading American pharmaceutical
contract development and manufacturing organization (CDMO),
and Antheia, the pharmaceutical ingredients manufacturer transforming essential
medicine supply chains, announced an ongoing partnership to onshore production
of essential medicines and establish more efficient, agile, and resilient
pharmaceutical supply chains in the United States.
⬥︎ In February 2025, SK Pharmteco Cell
& Gene Europe, a global contract development, manufacturing, and
analytical testing organization serving the pharmaceutical and cell & gene
therapy industries, along with Assistance Publique and Institut Imagine,
announced the signing of a contract for the production of a 200L CGMP clinical
batch of lentiviral
vector (LVV) and associated regulatory support.
Recently Advanced One-Stop-Shop Solution
Provided by the CDMO: Market’s Largest Potential
Integrated CDMOs offer a comprehensive
one-stop-shop strategy, covering everything from cell line development and
process characterization to commercial manufacturing and regulatory filing
support. This approach reduces delays caused by tech transfers between vendors.
Such end-to-end services and one-stop shop contract organizations remain
appealing to drug sponsors looking for speed, scalability, and strong consumer
support. They streamline the whole process, from early discovery and
development to commercial manufacturing and distribution, driving the growth in
the active
pharmaceutical ingredients CDMO market.
Active Pharmaceutical Ingredients CDMO
Market Report Scope
Report Attribute
Details
Market Size in
2025
USD 139.33 Billion
Market Size by
2034
USD 273.92 Billion
Growth Rate From
2025 to 2034
CAGR of 7.8%
Base Year
2024
Forecast Period
2025 to 2034
Segments Covered
Product, Synthesis,
Drug, Application, Workflow, Region
Market Analysis
(Terms Used)
Value (US$
Million/Billion) or (Volume/Units)
Report Coverage
Revenue forecast,
company ranking, competitive landscape, growth factors, and trends
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8371
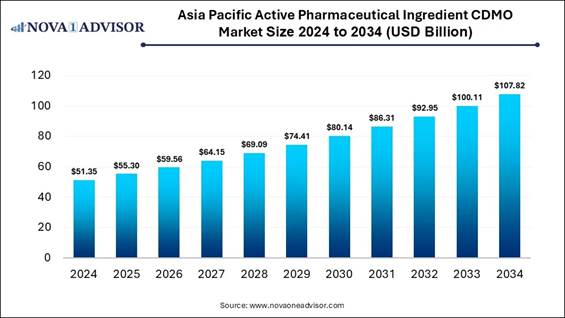
Asia Pacific Active Pharmaceutical
Ingredients CDMO Market Size and Trends 2025 to 2034
The Asia Pacific active pharmaceutical
ingredients CDMO market size was valued at USD 51.35 billion in 2024 and is
projected to surpass around USD 107.82 billion by 2034, registering a CAGR of
7.7% over the forecast period of 2025 to 2034.
North America dominated the active
pharmaceutical ingredients CDMO market in 2024, due to its advanced
manufacturing solutions, such as advancing complex, high-value APIs, including
HPAPIs and biologics. CDMOs in North America have developed techniques to
handle these substances safely and effectively. Also, the presence of a strong
government environment and high R&D spending drives the growth of the
market.
⬥︎ For Instance, In July 2025, ESTEVE
acquires Regis Technologies, a United States-based Contract Development and
Manufacturing Organization (CDMO),
headquartered in Chicago. This strategic move allows ESTEVE CDMO a physical
presence in the United States, expanding its contract development and
manufacturing solutions for innovative small-molecule active
pharmaceutical ingredients (APIs) across the entire drug development
lifecycle
The U.S. has a high presence of pharmaceutical
and biotechnology organizations and is increasing for adoption of novel
manufacturing processes. This drives consistent demand for CDMO solutions to
help a strong pipeline of new and complex drug molecules.
Why is Asia Pacific the Fastest Growing
in the Active Pharmaceutical Ingredients CDMO Market?
APAC is the fastest-growing region in the
market, with lower healthcare manufacturing expenses, the presence of a huge,
skilled workforce, strong government spending and policies in major countries
such as China and India, a massive API production infrastructure, and growing
worldwide demand for generic and affordable medicine solutions, which drives
the growth of the market.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8371
Active Pharmaceutical Ingredients CDMO
Market Segmentation Analysis:
By Type Analysis:
The synthetic
APIs segment dominates in the active pharmaceutical ingredients CDMO
Market, as manufacturing of synthetic APIs is usually more cost-efficient. They
provide advantages from low-cost raw materials, effective scaling, and
streamlined manufacturing processes, making drugs such as aspirin and
paracetamol highly affordable. Synthetic APIs rely on chemical inputs that are
broadly available, constant in price, and effortlessly sourced from worldwide
suppliers. Raw
materials for synthetic drugs are generally shipped to centralized facilities,
and the end products often remain stable at room temperature, making both
storage and transportation simpler.
On the other hand, the biotech
APIs segment is expected to grow significantly during the forecast
period, as biotech API manufacturing involves single-use
bioreactors; these systems are composed of plastic material, which is
sealed and sterilized by gamma radiation. Single-use bioreactors significant
device in the production of biotech API and HPAPI.
It is advantageous for both downstream and upstream processes. They allow the
development of targeted and biologic medicines, like vaccines and antibody
therapies.
By Drug Insights Analysis:
The generic APIs
segment dominated the market in 2024, as it makes medical care more affordable
and accessible. Generic APIs contribute to healthier communities and stronger
economies. Simple to enhance when application functionality is added, and there
is one single place to make modifications and publish novel API contracts.
Generic API is а one-size-fits-all, trying hard to accommodate the requirements
of each customer.
On the other hand, the innovative drugs
segment is expected to grow at the fastest CAGR in the market during the
forecast period, as APIs are the backbone of innovative medicine manufacturing,
forming significant components in drug formulations that manage, treat, and prevent
diseases.
By Application Analysis:
The Oncology segment
dominated the market in 2024, as high-potency active pharmaceutical ingredients
(HPAPIs) are considered significant in the production of oncology drugs,
enabling the production of personalized
cancer therapies. These API deliver efficient treatment at minimum
doses, making them important for reducing adverse effects while maximizing
therapeutic effect. This quality has caused a significant increase in the
demand for HPAPI manufacturing in oncology.
On the other hand, the cardiovascular
disease and diabetes APIs segment is expected to grow at the fastest
CAGR in the market during the forecast period, as API play an important role in
cardiology and diabetology drug manufacturing. Glimepiride, Glipizide,
Indapamide, and Atorvastatin have been discovered to have their unique
contributions to treating diabetes and heart disease. These APIs provide
targeted action with variable durations of control. Its longer effect ensures
sustained cholesterol control, lowering the challenges of plaque buildup in
arteries and defensive the heart from possible damage.
By Workflow Analysis:
The commercial
manufacturing segment dominated the market in 2024, as this segment has a
large-scale production strength of active pharmaceutical ingredients. It is
significant to offer consistent, quality, and compliant products available for
commercial distribution. It offers advantages like reduction of cost through
economies of scale and well-organized processes, access to advanced technology,
and faster time-to-market through rationalized production.
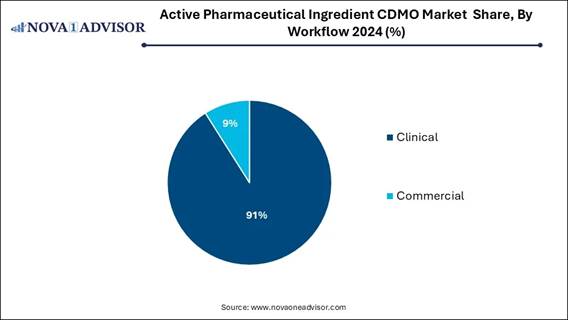
On the other hand, the clinical development
manufacturing segment is expected to grow at the fastest CAGR in the market
during the forecast period, as it offers significant benefits for
pharmaceutical organizations, particularly during the critical initial stages
of drug development.
Active Pharmaceutical Ingredients CDMO
Market Companies:
• Recipharm AB
• Thermo Fisher Scientific
Inc. (Pantheon)
• CordenPharma International
• Lonza
• Boehringer Ingelheim International GmbH
What is Going Around the Globe?
⬥︎ In April 2025, Sumitomo Chemical
established a new company, Sumitomo Chemical Advanced Medical Solutions America
LLC, in Marlborough, Massachusetts, the United States. This company will serve
as a CRO for Sumitomo Chemical’s Oligonucleotide CDMO business.
⬥︎ In May 2025, Lonza, a contract development and
manufacturing organisation (CDMO), announced the launch of its new
Design2Optimize platform to enhance process development and manufacturing of
small molecule APIs.
⬥︎ In October 2024, Thermo Fisher Scientific
launched Accelerator Drug Development, described as the company’s 360° contract
development and manufacturing organization and contract research organization
drug development solutions, as part of its showcase at CPHI Milan.
You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344
Related Report –
🔹Biopharmaceutical CDMO Market- https://www.novaoneadvisor.com/report/biopharmaceutical-cdmo-market
🔹Biologics CDMO Market- https://www.novaoneadvisor.com/report/biologics-cdmo-market
🔹Oligonucleotide CDMO Market- https://www.novaoneadvisor.com/report/oligonucleotide-cdmo-market
🔹Cell And Gene Therapy CDMO Market- https://www.novaoneadvisor.com/report/cell-and-gene-therapy-cdmo-market
🔹U.S. Active Pharmaceutical Ingredients CDMO Market- https://www.novaoneadvisor.com/report/us-active-pharmaceutical-ingredients-cdmo-market
🔹North America Topical Drugs CDMO Market- https://www.novaoneadvisor.com/report/north-america-topical-drugs-cdmo-market
🔹Topical Drugs CDMO Market- https://www.novaoneadvisor.com/report/topical-drugs-cdmo-market
🔹U.S. Pharmaceutical CDMO Market- https://www.novaoneadvisor.com/report/us-pharmaceutical-cdmo-market
🔹Pharmaceutical CDMO Market- https://www.novaoneadvisor.com/report/pharmaceutical-cdmo-market
Active Pharmaceutical Ingredients CDMO
Market Report Segmentation
This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each of
the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has
segmented the Active Pharmaceutical Ingredients CDMO market.
By Product
• Traditional Active Pharmaceutical
Ingredients (Traditional API)
• Highly Potent Active Pharmaceutical
Ingredients (HP-API)
• Antibody Drug Conjugate (ADC)
• Others
By Synthesis
• Synthetic
• Biotech
By Drug
• Innovative
• Generics
By Workflow
• Clinical
• Commercial
By Application
• Oncology
• Hormonal
• Glaucoma
• Cardiovascular disease
• Diabetes
• Others
By Region
• North America
• Europe
• Asia-Pacific
• Latin America
• Middle East & Africa (MEA)
Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/8371
About-Us
Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision
medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn
