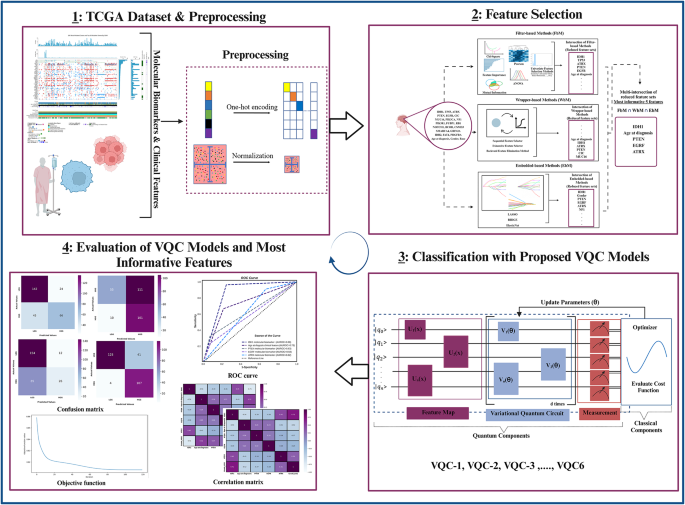
In this section, the results obtained with the ensemble feature selection method and VQC models are discussed in detail. In this context, comparisons are made with studies in the literature. First, the results of the ensemble feature selection process are presented and compared with the findings in the literature. Next, the details and circuit structure of the VQC-1 model, which demonstrated the best classification performance among the six proposed VQC models, are explained. Subsequently, the classification performances of the five most important features in distinguishing LGGs from HGGs are examined, and the molecular and clinical significance of these features is evaluated. Finally, the classification performances of 6 different classical ML models, obtained using 5-fold cross-validation, are analyzed. The 5-fold cross-validation results of the VQC-1 model are compared with both traditional models and similar studies in the literature.
Feature selection
In this section, of the study, the role of the ensemble-based feature selection method is emphasized in the discovery of molecular biomarkers and clinical features for classifying LGGs and HGGs based on TCGA data. As evidenced by numerous studies in the literature93, many tumors do not arise from a single gene mutation but rather result from a series of genetic alterations. Therefore, the identification of significant molecular biomarkers enables a more accurate correlation of gene expression with disease incidence. Moreover, in the early stages, the identification and selection of significant clinical features alongside these genetic molecular biomarkers are extremely critical in distinguishing between LGGs and HGGs. Additionally, the determination of crucial features significantly impacts the ultimate performance of classifiers. For example, in this study109, feature selection techniques were used for biomarker discovery in four cancer diagnosis microarray datasets: leukemia, colon, lymphoma, and prostate. To ensure the stability of biomarker identification and enhance classification performance in the subsequent stage, an ensemble feature selection method was proposed. These authors demonstrated that the robustness of support vector machines (SVMs) for biomarker discovery could be significantly increased by employing ensemble feature selection techniques while simultaneously improving classification performance. With the proposed methodology, an increase of almost 0.3 in the robustness of the selected biomarkers, along with an improvement of approximately 0.15 in classification performance, was observed across the 4 microarray datasets. In another study110, ranking-based feature selection techniques and state-of-the-art feature selection methods were applied to 6 different DNA microarray datasets, namely, Breast, Colon, Leukemia, Lymphoma, Lung, and Ovarian. The effectiveness of these methods was determined using 4 different classifiers. Overall, significant improvements in classification results were observed as the number of features decreased for each dataset compared to using the entire feature set. This demonstrates that the application of feature selection techniques substantially enhances classification performance. Furthermore, in all experiments, it was observed that even without feature selection, the RFC yielded the most favorable outcomes. Identifying the most informative molecular biomarkers and clinical features and finding small feature sets with high classification accuracy is crucial both for minimizing the cost of classification and for biological insight. In many studies, such as111,112,113, classical feature selection methods, such as filter, wrapper, and embedded techniques, have been individually emphasized for their positive contributions to classification outcomes. However, there is also a tendency to combine algorithms, as in the case of hybrid, voting-based or ensemble methods, which usually combine two or more feature selection algorithms of different conceptual origins in a sequential manner. Given the complexity and noise in genomic data, obtaining a perfect solution and identifying important features with a single feature selection algorithm can be quite challenging. Therefore, in this article, we used an ensemble feature selection method to evaluate different profiles of the data and to identify informative marker genes and clinical features that facilitate the distinction between LGGs and HGGs. We identified the 5 most significant features by the proposed ensemble-based model: IDH1, age at diagnosis, PTEN, ATRX, and EGRF. The main reasons for selecting these 5 features are their ability to yield the best classification accuracy, consume less processing time, and require fewer memory configurations than the others. These results are consistent with the findings of previous studies, including various research conducted in the fields of neuroscience and neuro-oncology26,114,115,116,117. In this study26, with the aim of enhancing glioma grading performance using TCGA and CGGA glioma datasets, a novel hybrid feature selection method was first proposed. Subsequently, five supervised learning models were employed to investigate the impact of feature selection methods on classification performance. The proposed method, called “GradWise”, comprises a rank-based weighted hybrid filter and embedded feature selection techniques aimed at identifying significant molecular markers and clinical features in the TCGA and CGGA glioma datasets. The SVM classifier yielded the most favorable outcomes, achieving an accuracy of 87.007% for the TCGA cohort and 80.412% for the CGGA cohort when utilizing 13 features. For the TCGA dataset, the selected features for the best result included ‘CIC’, ‘Age’, ‘IDH1’, ‘PTEN’, ‘ATRX’, ‘PIK3R1’, ‘NF1’, ‘IDH2’, ‘GRIN2A’, ‘NOTCH1’, ‘TP53’, ‘EGFR’, and ‘MUC16’. Moreover, for the CGGA dataset, the best identified features were IDH1, Age, PTEN, PDGFRA, and NF1. The obtained features align with the most informative features identified in our research. In another study86, a novel hierarchical voting-based methodology was suggested to improve both the identification of significant features and the classification performance of ML models. The methodology created using TCGA and CGGA glioma datasets consists of a combination of voting-based feature selection methods derived from four different feature selection methods (weight of evidence, recursive feature elimination, random forest (RF), LASSO) and the soft voting-based ensemble learning methodology generated from five supervised learning models (LR, SVM, KNN, RF, AdaBoost). The results obtained from the hierarchical voting-based methodology were individually compared with those obtained from the LASSO feature selection method. Consequently, in the TCGA glioma dataset, the highest accuracy of 0.876 was attained through the utilization of SVM, RF, and AdaBoost in combination. On the other hand, for the CGGA glioma dataset, the most favorable accuracy of 0.797 was achieved by employing the combination of SVM, KNN, RF, and AdaBoost.
Classification performance of VQC models
In this part of the study, by changing the hyperparameters of VQC, such as feature maps, PQC structures and optimization methods, an attempt was made to obtain a circuit design that has minimal quantum gate and circuit depth. The fidelity of modern quantum computation is negatively affected by increasing the number of qubits or gates. Moreover, the training time for quantum circuits can be significantly prolonged by considering phenomena such as barren plateaus118. Additionally, the intricacies of designing architectures, selecting optimization functions, and initializing parameters are far more complex and nuanced than one might superficially assume. Therefore, a VQC with optimal hyperparameters not only reduces the training time but also enables us to avoid phenomena such as barren plateaus. In addition, preprocessing steps conducted before the application of VQC significantly improve the classification performance.
For instance, in this study119, an attempt was made to predict dementia in elderly patients using IBM’s VQC model within the Qiskit framework. The performance of this model was compared with that of a classical SVM with a linear kernel considering different numbers of features. In the preprocessing stage before the VQC model, a min–max normalization process was applied, followed by the selection of the most important features. The variable ranking process was carried out based on the scores provided by classifiers using the gradient boosting, RF, extra trees, and K-best ML methods. In the present study, when 3 features related to dementia were utilized, the accuracy of the VQC model approached 0.9, whereas the performance of the SVM with linear kernel model was approximately 0.82. Despite the emphasis on the superiority of the VQC model in the study, no information was provided regarding the gates used within the VQC. In another study120, the proposed VQC model was applied to solve binary classification problems on a synthetic dataset, as well as real datasets obtained from the University of California Intelligence Machine Learning (UCI), including a sonar dataset and a diabetes dataset. The performance of the suggested VQC model was compared with that of other methods, such as SVM and QSVM. Within the parameterized quantum circuit of VQC, \(\:{R}_{y}\), \(\:{R}_{z}\:\)rotational, and CX gates were utilized. To enhance the classification performance, a preprocessing step was introduced. In this step, classical ML methods, namely, RF, LR, and SVM, were employed for feature ranking. Eight important features were identified for each dataset based on these methods. After applying min–max normalization, the features were encoded from the classical space to the quantum Hilbert space using basis and amplitude encoding methods. As a result, the amplitude encoding-based VQC achieved accuracies of 0.984, 0.6730 and 0.7450 on the synthetic, sonar, and diabetes datasets, respectively. This method successfully improved the prediction rates for both the synthetic and diabetes datasets, but its impact on the sonar dataset was limited. In this study121, a two-stage approach was proposed to classify and subsequently segment brain tumors at the initial stage, aiming to improve patient survival rates. The methodology is designated as Phase 1 and Phase 2 and was tested on three different types of benchmark datasets, namely, TCGA, BRATS 2020, and locally collected images. In Phase 1, the Inceptionv3 model was utilized to extract significant features from brain MR images. The values of the score vectors obtained from the softmax function in the final layer of this pretrained model were employed to classify the brain tumors. These score vectors were then transmitted to a VQC with a 4-qubit structure, for model training. The parametrized circuit included \(\:{R}_{x}\), \(\:{R}_{y}\), and \(\:{R}_{z}\) rotational gates, as well as CX gates. The Kaggle dataset achieved accuracy rates of 0.9944 for no tumor, 0.9925 for meningioma, 0.9803 for pituitary tumors, and 0.9934 for glioma. With respect to classifying tumor and nontumor tumor types, the proposed method achieved an accuracy of 0.9333 on locally collected images. For the 2020-BRATS challenge, the proposed method achieved an accuracy of 0.9091 in differentiating HGGs from LGGs. In Phase 2, to analyze the total infected area postclassification, a modified Seg-Network was introduced, providing global accuracies of 0.982, 0.999, and 0.997 on the Kaggle dataset, locally collected images, and 2020-BRATS Challenge, respectively. Furthermore, in the literature, it has been indicated in numerous studies that hybrid methods enhance the performance of quantum ML techniques. In certain studies, although the performance advantage of the proposed hybrid classical and quantum methods over classical methods is emphasized when applied to problems such as brain tumor classification122, automatic Alzheimer’s diagnosis123, brain disorder classification124, and diabetic foot ulcer classification125, some research has highlighted the advantage of rapid convergence provided by hybrid models, leading to a shortened processing time, even when the performance is equivalent126.
In our study, after identifying the most informative molecular markers and clinical features through the ensemble feature selection process (as described in “Feature selection”, six different VQC models with different hyperparameters were designed using these features. Among these models, the VQC-1 model, which employs \(\:{R}_{x}\), rotational and CX gates in the feature map and \(\:{R}_{y}\), \(\:{R}_{z}\) and CY gates in the parameterized quantum circuit, along with the AQCD optimization method for parameter updates, achieved the highest accuracy in distinguishing between LGGs and HGGs. Here, single-qubit rotational gates (\(\:{R}_{x}\), \(\:{R}_{y}\) and \(\:{R}_{z}\)) are employed to rotate each data qubit in a specific manner for a given class label (LGGs or HGGs), while in two-qubit quantum gates (CX, CY\(\:)\:\)the quantum entanglement phenomenon is leveraged to ensure that the rotation of each data qubit affects all other data qubits in a specified manner, involving them in instantaneous interactions with all possible states of the feature vector. In addition, the features in the TCGA glioma dataset utilized in our study consisted entirely of categorical data, excluding age at diagnosis. Quantum state preparation is crucial for establishing a functional flow in a quantum ML model and serves as a significant preprocessing step in VQC. After converting categorical data into a series of binary variables through one-hot encoding (“Preprocessing”), \(\:{R}_{x}\) rotational and CX gates were employed during the quantum state preparation step.
In addition to these results, before employing the VQC-1 model, we utilized principal component analysis (PCA), which is the most widely used feature extraction method in quantum ML literature. We conducted a performance comparison with our proposed hybrid classical and quantum computing model, demonstrating its impact on the classification results. When PCA was used, the accuracy of the VQC-1 model in distinguishing between LGGs and HGGs was 0.72. Although this accuracy is 0.272 lower than that of our proposed model, many studies in the literature have emphasized the weakness of the PCA method in terms of feature selection90. While PCA may provide a speed advantage to quantum ML methods, it renders data interpretation and readability impossible and is not a favorable approach in terms of transparency90,127. These findings indicate that our proposed hybrid model not only enhances classification performance but also provides more interpretable results.
Classification performance of most informative features and correlation analysis between most informative features and glioma tumor grade
Molecular analysis of tumors has significantly impacted the diagnosis and classification of gliomas, yielding substantial implications for both prognosis and treatment guidance. The emergence of DNA methylation studies has provided increased knowledge about specific genetic changes in glioma genomes, facilitating accurate monitoring of glioma patients and paving the way for personalized therapies. Since the inclusion of molecular markers in the WHO classification in 2016 and significant changes in 2021 advancing the role of molecular diagnosis in CNS tumor classification, numerous studies have been conducted to identify crucial molecular markers facilitating the diagnosis and differentiation of glioma tumors. In this section, of our study, we evaluated the performance of the most informative features mentioned in “Feature selection”, namely, IDH1, age at diagnosis, PTEN, ATRX, and EGFR, in differentiating between LGGs and HGGs. The features were tested for their individual predictive values. To assess the performance of each significant feature, the VQC-1 model, as described in “Classification performance of most informative features andcorrelation analysis between most informative features and gliomatumor grade”, was employed. Among these five important features, IDH1 exhibited the highest performance in differentiating between LGGs and HGGs, with an accuracy of 0.84.
As highlighted in numerous recent studies17,20,128,129,130, the IDH1 molecular biomarker has been defined as a significant prognostic factor and molecular diagnostic criterion for investigating the behaviors of LGGs and their subtypes. IDH1 is an enzyme that catalyzes the oxidative decarboxylation of isocitrate to a-ketoglutarate. This enzyme plays a crucial role in regulating metabolic pathways essential for the normal functions of cells. Approximately 65–90% of LGGs exhibit a mutation in IDH1, and this mutation in LGGs results in an increase in genome-wide DNA methylation. The hypermethylated phenotype observed in LGGs with IDH1 mutation has been correlated with an enhanced survival rate. In studies5,131, it has been emphasized that IDH1 mutation is almost never found in HGGs, and the commonalities between LGGs without IDH1 mutation and HGGs have been highlighted in many aspects. These results underscore the significant contribution of the IDH1 molecular biomarker to the differentiation of LGGs from HGGs. In our study, Pearson’s correlation analysis revealed a significant negative correlation (r = − 0.71, p IDH1 molecular marker and HGG and a strong positive correlation (r = 0.71, p IDH1 molecular marker and LGG. The robust negative correlation between the IDH1 molecular marker and HGGs, as well as the strong positive correlation with LGGs, when well understood and interpreted, can aid in comprehending the genetic differences among tumors. These differences are associated with tumor behavior, prognosis, and treatment response. Indeed, mutations in the IDH1 gene are acknowledged as clinically significant marker for LGGs and are more frequently found in LGGs than in HGGs5,131,132. This finding underscores the utility of the IDH1 mutation as a crucial indicator in the clinical context, providing valuable insights into the genetic landscape of gliomas and their clinical implications.
The IDH1 molecular marker is followed by the only clinical feature among the five most informative features, namely, age at diagnosis, for which the accuracy was 0.74. Age at diagnosis is one of the most crucial clinical feature for distinguishing between LGGs and HGGs. LGGs tend to affect younger patients more frequently, with a median age at diagnosis of 447. Numerous studies emphasize that a younger age is often associated with mutational status. On the other hand, the majority of HGGs (approximately 90%) develop de novo in older patients. In many studies in the literature, from clinical information, only age at diagnosis has been used for estimating molecular markers133,134. For instance, in this study134, the molecular biomarker IDH and 1p19q codeletion status were predicted in gliomas using clinical information such as age, along with histogram, shape, texture features extracted from preoperative MR images of 538 glioma patients. MRI data obtained from the three institutions were used as the training cohort. The TCGA (for IDH) and TCIA (for 1p19q codeletion status) cohorts were used independently to evaluate the performance of the final model. The RF algorithm was specified as the prediction model with a tree depth of 64 and a maximum of 4096 trees. Additionally, the predictive value of each feature in determining the IDH genotype and 1p19q codeletion status was measured using the AUROC. As a result, when age and imaging features were used for IDH genotype prediction, the model yielded an AUROC of 0.921 in the training cohort and 0.919 in the validation cohort. For 1p19q genotype prediction, an AUROC of 0.685 was obtained from the training cohort, and 0.716 was obtained from the TCIA test cohort. In both cases, age had the highest predictive value. Within our study, Pearson’s correlation analysis revealed a moderate positive correlation (r = 0.52, p age at diagnosis and HGG and a moderate negative correlation (r = − 0.52, p age at diagnosis and LGG. These findings demonstrated the significance of age at diagnosis in distinguishing between LGGs and HGGs.
PTEN is the second molecular marker with the highest accuracy (0.65) among the five most informative features. PTEN, or “Phosphatase and Tensin Homologue”, regulates apoptosis (programmed cell death) and DNA damage repair. In brain tumors, particularly in frontal lobe GBM, there is a decrease in the expression of this gene, and PTEN loss is associated with GBM. As indicated in numerous studies, IDH-wildtype GBM (or HGGs) have mutations in the PTEN gene. Approximately 40% of GBMs harbor mutations in the PTEN gene protein, and approximately 70% exhibit heterozygous loss at the PTEN locus. For instance, in this study135, a clinic-genomic analysis was conducted using data from a total of 1477 glioma patients from TCGA and the Chinese cohort. The results obtained from the univariate survival analysis revealed an association between high-risk glioma patients (HGGs) with shortened survival and deletion of the PTEN gene. In our study, Pearson’s correlation analysis revealed a weak positive correlation (r = 0.37, p PTEN molecular marker and HGG and a weak negative correlation (r = − 0.37, p PTEN molecular marker and LGG. According to these results, although the deletion of the PTEN gene is highly important for distinguishing between LGGs and HGGs, it has been demonstrated that this gene is not a sufficient molecular marker for distinguishing LGGs from HGGs.
The PTEN molecular marker is followed by the EGRF molecular marker, with an accuracy of 0.65 in distinguishing between LGGs and HGGs. EGRF or epidermal growth factor receptor (EGFR) is, also known as the HER1/ErbB1 receptor, is a molecular marker for determining treatment response and prognosis in gliomas. EGFR is a significant activator influencing various signaling pathways and physiological responses, such as proliferation, survival, and tumorigenesis131. It is observed in 60% of primary GBMs and is often associated with HGGs17. Moreover, the detection of EGFR amplification has been indicated as a criterion for the diagnosis of GBM17. In some studies, different MRI sequences have been employed for the noninvasive determination of molecular subtypes of GBM, facilitating the detection of EGFR and EGFR mutations through these sequences136. In this study136, an attempt was made to identify EGFRvIII, the most common variant of EGFR and a significant molecular marker for distinguishing the molecular subtypes of GBM, using one of the parameters of magnetic resonance perfusion-weighted imaging, Relative Tumor Blood Volume (rTBV). The ROC curve analysis in the study demonstrated that the rTBV identified EGFRvIII with an accuracy of 0.81. Additionally, it has been indicated that the expression of vascular endothelial growth factor (VEGF) is associated with a higher relative tumor blood volume in patients with GBM. Within our study, Pearson’s correlation analysis revealed a weak positive correlation (r = 0.24, p EGFR and HGG and a weak negative correlation (r = − 0.24, p EGFR and LGG.
The fifth and final crucial feature was the ATRX molecular marker, which had an accuracy of 0.56 in distinguishing between LGGs and HGGs. ATRX, or alpha-thalassemia/mental retardation X-linked gene mutation, serves as a robust biomarker that can guide the diagnosis and prognosis of glioma patients. Mutations or losses in the ATRX gene are associated with the alternative lengthening of telomeres (ALT) phenotype41. This condition significantly influences the biological behaviors of astrocytic tumor cells and is correlated with the survival of patients with astrocytic tumors. Moreover, mutations in this gene are frequently found in LGGs, particularly in nearly 86% of LGGs with IDH mutations5. Several studies have highlighted the considerable potential of noninvasive methods for predicting ATRX in LGGs, offering diagnostic and prognostic insights. For instance, in this study41, a dataset pertaining to the ATRX mutation status of patients in the TCGA database was utilized for ATRX prediction in LGGs, with patients from the CGGA cohort serving as an external validation set. After radiomic features were extracted from T2-weighted magnetic resonance images, nine features were selected using the LASSO regression model out of 431 radiomic features. In the final stage, the SVM model was used to predict the ATRX mutation status in the training, validation, and external validation sets. As a result, AUC values of 0.94, 0.925, and 0.725 were achieved for each dataset, respectively. Within our study, Pearson’s correlation analysis revealed a weak negative correlation (r = − 0.31, p ATRX and HGG, as well as a weak positive correlation (r = 0.31, p ATRX and LGG.
In our study, while emphasizing the significance of the EGFR and ATRX molecular markers, the low correlation values between HGG and LGG grades for these markers (0.24 and − 0.24 for EGFR and − 0.31 and 0.31 for ATRX, respectively) can be attributed to several main reasons:
-
1.
Correlation values can be influenced by the complex biological processes underlying gliomas. Molecular markers such as EGFR and ATRX may not be directly linked to tumor grade, and the observed correlations may be influenced by other factors in these complex processes.
-
2.
Gliomas exhibit significant genetic diversity, and the impact of EGFR and ATRX mutations on tumor grade may vary among different subtypes. For instance, the ATRX biomarker is often used more for distinguishing LGG subtypes than for differentiating between LGGs and HGGs.
-
3.
The size of the dataset used also affects the correlation values. A larger and more diverse dataset could provide a more comprehensive representation of the relationship between EGFR or ATRX and glioma grade.
-
4.
The temporal aspects of tumor development and progression may not be fully reflected in static measurements of molecular markers. Changes in the expression of EGFR and ATRX over time may lead to fluctuations in correlation values with tumor grade.
Finally, although the IDH1 molecular marker is frequently used alone for distinguishing between LGGs and HGGs, the results obtained in our study suggest that, in addition to IDH1, the inclusion of other mutations (PTEN, EGFR, and ATRX) and other clinical information (age at diagnosis) may be more beneficial for glioma classification.
Classification performance of classical models
The performance of the VQC-1 model, which exhibited the best classification results among six different VQC models proposed with various hyperparameters, was compared with six different classical ML algorithms using a five-fold cross-validation method. The classification accuracy values obtained for the classical ML models during this process are as follows: KNN − 0.82, SVC − 0.75, DTC − 0.80, RFC − 0.82, XGBoost − 0.84, and GBM − 0.83. Furthermore, VQC-1 achieved 0.83 accuracy under the same cross-validation setup. These results demonstrate that VQC-1 performs comparably to classical ensemble methods such as XGBoost and GBM, while outperforming KNN, SVC, DTC, and RFC in terms of classification accuracy. These findings indicate that VQC-1 is not only competitive with classical models but also exhibits superior performance in comparison to certain models. This suggests that hybrid classical and quantum computing models may offer promising alternatives for biomedical classification problems. The competitive performance of the proposed hybrid classical and quantum computing model, comparable to classical models, aligns with previous studies showing the effectiveness of ML-based approaches for glioma diagnosis and classification. In the literature, various classical ML models have been used to improve the detection and classification of glioma tumors. For instance, in this study116, a classification model was developed to identify patients classified as LGG or GBM and to improve clinical decision support processes by considering clinical and molecular/mutational factors using data from The Cancer Genome Atlas (TCGA). The model integrates feature selection methods (Pearson correlation, Mutual Information, and PCA) with various ML-based classification algorithms, including Random Forest, Decision Trees, Logistic Regression, KNN, AdaBoost, SVM, CatBoost, LGBM, and XGBoost, as well as deep learning models (ANN and CNN). An ensemble stacking approach was also employed, and the Final Stack Model demonstrated strong performance. The XGBoost model achieved the highest performance, obtaining 88% accuracy, 82% precision, 94% recall, and 92% AUC. In addition, the Final Stack model achieved 82% accuracy, 79% precision, 85% recall, and 90% AUC. To enhance the model’s transparency and clinical applicability, four different explainable artificial intelligence (XAI) techniques (SHAP, LIME, ELI5, and QLattice) were employed. These methods analyzed the decision-making mechanisms of the model and identified the most critical features in glioma diagnosis. The findings indicate that genetic factors such as IDH1, PIK3CA, ATRX, PTEN, CIC, EGFR, TP53, as well as the age at diagnosis, play a crucial role in glioma classification.
Likewise, the study117 employed the same TCGA dataset to propose a data-centric approach for the accurate prediction and grading of gliomas. To examine the effects of oversampling and limited undersampling algorithms, six ML models (KNN, SVM, multilayer perception—MLP, LR, and two ensemble models, RF and CatBoost) were employed. Additionally, to identify the most informative molecular biomarkers and clinical features, four feature ranking algorithms (information gain, Gini index, chi-square, and random forest) were applied to the normalized data. Using a multiple intersection approach, the most informative features were determined as IDH1, age at diagnosis, PTEN, CIC, and ATRX. Furthermore, the explainability of the predictions was analyzed using global feature importance and SHAP methods, demonstrating that IDH1 and age at diagnosis had the greatest influence on model decisions. As a result, the two ensemble models (RF and CatBoost) exhibited superior performance compared to the other models. Moreover, it was found that the oversampling process applied to the minority class using the SMOTE method improved the predictive performance of the models.
In conclusion, the maximum accuracy obtained when using the five features selected by the ensemble feature selection method (0.74) and the accuracy value obtained through five-fold cross-validation (0.83) indicate that the VQC-1 model demonstrates better generalization when evaluated across multiple training and validation splits. In other words, the model effectively captures all variations within the dataset, exhibiting a more reliable and robust generalization capability. This result suggests that hybrid classical and quantum computing models, due to their strong generalization abilities, may provide more reliable and effective solutions compared to classical models in biomedical applications with complex and highly correlated data, including glioma tumors.