Adaptive neuromorphic hardware design using reconfigurable ZnPS3 memristors
The human brain, with its 1011 neurons interconnected by 1015 synapses, functions as a highly complex and adaptable system capable of continuous self-reconfiguration3. Neurons serve as the primary elements for processing information and exhibit over 20 distinct dynamic responses to electrochemical signals influenced by both past activity and environmental stimuli, enabling efficient temporal information processing29. Synapses, on the other hand, store information by modulating the strength of connections between neurons—a process known as synaptic plasticity—which underpins the memory and learning functions of brain30. Neurons specialized in various functions work in tandem with synapses, allowing the brain to execute a wide array of sophisticated tasks, including object recognition, pattern recognition, language processing, and adaptive learning, with remarkable speed and efficiency31,32,33,34.
Adaptive neuromorphic computing aims to emulate the functionality of brain, efficiency, and adaptability in hardware by dynamically adjusting the behavior of artificial neurons and synapses29. Reconfigurable memristors, which can switch between volatile and non-volatile memristive states, are particularly well-suited to mimic the diverse dynamics of neurons and synapses. ECM-based reconfigurable memristors, utilizing Ag conductive filaments with high surface-to-volume ratios, offer variable filament lifetimes ranging from microseconds to years9, making them promising candidates for adaptive neuromorphic systems. Our design employs a ZnPS3 switching layer that facilitates controlled Ag ion migration. By applying distinct electrical schemes, we can regulate Ag ion migration to create either thin or thick conductive filaments. Thin filaments tend to rupture spontaneously, enabling volatile switching, while thick filaments remain stable, allowing for non-volatile switching, as demonstrated in Fig. 1a.
Fig. 1: Design of adaptive neuromorphic computing using reconfigurable ZnPS3 memristors.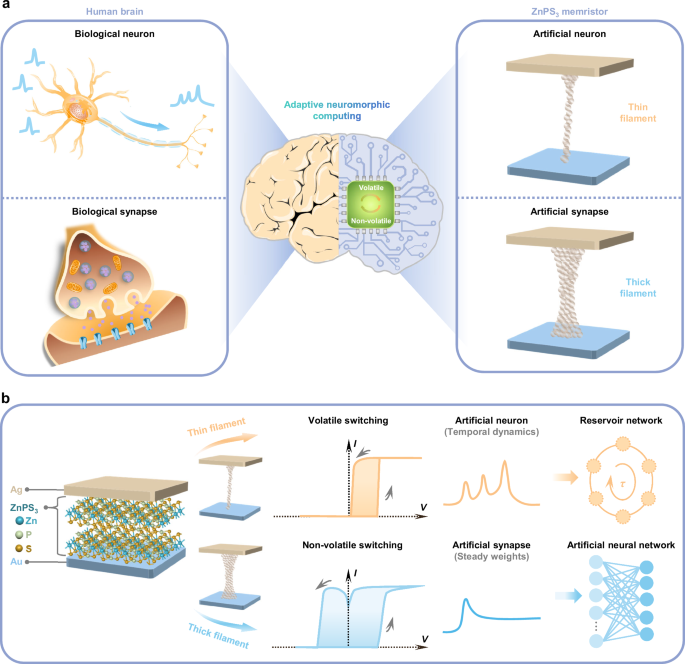
a Conceptual illustration of adaptive neuromorphic computing using reconfigurable ZnPS3 memristor, with controlled Ag ion migration to emulate biological neurons and synapses. b Schematic of the ZnPS3 memristor structure, along with illustrations highlighting its ability to mimic neuron-like temporal dynamics with higher-order complexity for reservoir networks and synapse-like adjustable weights for fully connected artificial neural network (ANN).
The ZnPS3 memristor utilizes a vertical stack configuration of Ag/ZnPS3/Au, as illustrated in Fig. 1b. Its optical image is provided in Supplementary Fig. 5. This device exhibits unipolar volatile switching, characterized by a memristive loop at high positive bias and a return to the off state at zero bias under low compliance current. Intriguingly, the device also demonstrates bipolar non-volatile switching, where the on state persists at zero bias following a set operation under high compliance current. The volatile switching enables efficient nonlinear mapping of time-varying electrical pulse inputs into a high-dimensional feature space, producing neuron-like temporal dynamics with complex, higher-order characteristics. These properties make ZnPS3 memristors suitable as artificial neurons within physical reservoir networks. Conversely, the non-volatile switching allows ZnPS3 memristors to store adjustable weights (conductance states) and maintain long-term memory, akin to synapses, facilitating fully connected ANNs. By leveraging the same device structure, ZnPS3-based memristors combine the functionalities of both artificial neurons and synapses, thereby simplifying fabrication, optimizing chip area, and streamlining neuromorphic hardware design. The flexibility and adaptability of reconfigurable ZnPS3 memristors make them well-suited for a range of neural network architectures, establishing them as versatile platforms for adaptive neuromorphic computing.
Reconfigurability and underlying mechanisms of ZnPS3 memristor
Figure 2a, b illustrate the representative current-voltage (I-V) characteristics of a ZnPS3 memristor with direct voltage sweeping on its Ag electrode under different compliance currents, demonstrating distinct resistive switching behaviors. At a low compliance current of 1 μA, the ZnPS3 memristor demonstrates unipolar volatile resistive switching (Fig. 2a). Initially, the memristor is in a high resistance state (HRS). When the applied voltage reaches the threshold voltage (Vth), the device switches to a low resistance state (LRS), with its current rapidly reaching the compliance limit. Upon reducing the voltage to the hold voltage (Vhold), the device spontaneously returns to the HRS, indicated by a sharp drop in current. At a higher compliance current of 500 μA, the memristor exhibits bipolar non-volatile resistive switching (Fig. 2b), where the device switches from its HRS to LRS at the positive set voltage (Vset) and returns to the HRS at the negative reset voltage (Vreset). Further investigation reveals that this resistive switching behavior is absent when the active Ag electrode is replaced with an inert Au electrode (Supplementary Fig. 6), indicating that Ag ion migration under an electric field is responsible for the switching in the Ag/ZnPS3/Au memristor. This hypothesis is supported by AFM and CAFM analyses (Supplementary Fig. 7), which reveal significant morphology and current changes attributed to Ag migration, thus confirming the ECM mechanism in the Ag/ZnPS3/Au memristor. The different resistive switching behaviors observed at varying compliance currents are attributed to the size-dependent stability of the Ag conductive filaments. Low compliance current results in the formation of thin, unstable Ag filaments that spontaneously break due to atomic surface diffusion driven by system energy minimization (Fig. 2c)9, leading to volatile switching. In contrast, higher compliance currents create thicker, more stable Ag filaments with extended lifetimes (Fig. 2d), resulting in non-volatile switching.
Fig. 2: Reconfigurability and underlying mechanisms of ZnPS3 memristor.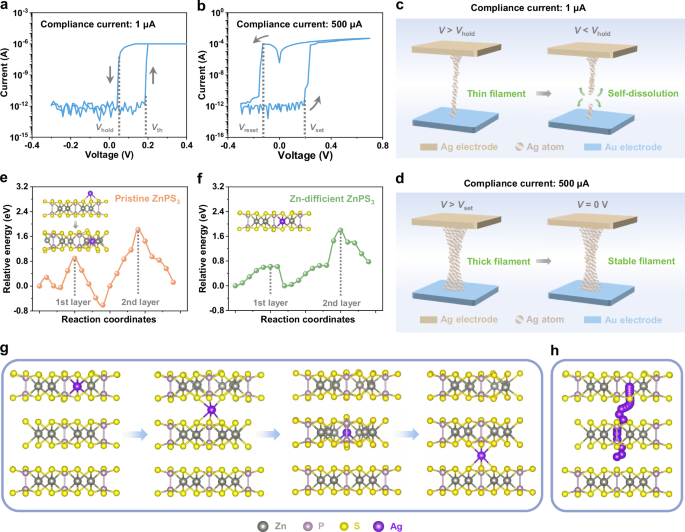
a, b I-V curves of the ZnPS3 memristor under compliance currents of 1 μA (a) and 500 μA (b). c, d Schematic diagrams showing the evolution of conductive filaments under compliance currents of 1 μA (c) and 500 μA (d). e, f Calculated diffusion energy barriers for Ag ion migration in pristine (e) and Zn-defective (f) ZnPS3. g Illustration of Ag ion migration within Zn-defective ZnPS3. h Pathway of Ag ion migration in Zn-defective ZnPS3.
We further investigate the reconfigurability of the ZnPS3 memristor using electrical pulse stimuli. During the pulse measurements, voltage pulses with amplitudes of 0.5 and 1.0 V are applied to the Ag electrode for a short duration of 2 μs, with a low-amplitude pulse of 0.01 V used to monitor the resistance states of the device. As shown in Supplementary Fig. 8a, applying a lower-amplitude pulse of 0.5 V causes the memristor to switch on due to the formation of Ag filament, indicated by an immediate increase in current. After the pulse is removed, the current gradually declines, and the device reverts to its initial HRS as the Ag filament dissolves, demonstrating volatile switching behavior. In contrast, applying a higher-amplitude pulse of 1.0 V to the Ag electrode (Supplementary Fig. 8b) produces an abrupt increase in current that remains stable after the pulse ends, signaling a transition to a stable LRS and displaying non-volatile behavior.
This reconfigurability via voltage pulse stimuli allows the memristor to respond to pulse signals with distinct dynamics, resembling the way biological neurons and synapses react to external stimuli, thereby enabling emulation of biological systems. Specifically, biological neurons are characterized by dynamic nonlinear responses and short-term (fading) memory, behaviors that can be emulated by the ZnPS3 memristor. Under weak voltage pulses, the device exhibits a nonlinear increase in current followed by a gradual decay once the stimulus is removed, effectively mimicking neuronal temporal dynamics. Similarly, synaptic plasticity—manifested in biological systems as long-term potentiation (LTP) and long-term depression (LTD)—can be replicated in the ZnPS3 memristor through the controlled formation and dissolution of robust Ag filaments induced by sustained voltage pulses of opposite polarities. The detailed mechanisms underlying these neuromorphic behaviors will be elaborated in subsequent sections.
In ECM memristors with vdW single-crystal switching layers, an initial electroforming process is typically required to create pathways, such as grain boundaries and vacancies, to enable metal ion migration for subsequent reversible resistive switching35,36. However, our ZnPS3 memristors display electroforming-free resistive switching behavior (Supplementary Fig. 9), motivating an investigation into the underlying mechanisms. To explore this, we conducted density functional theory (DFT) simulations using a three-layer ZnPS3 model to elucidate Ag ion migration processes. Our focus was on vacancies, given their role as preferential diffusion pathways for active metal ions in single-crystal switching materials37. The DFT simulations identified sulfur vacancies (Vs) and zinc vacancies (VZn) as the most energetically favorable defects within ZnPS3 (Supplementary Fig. 10). Additionally, XPS analysis confirmed the presence of Vs and VZn in ZnPS3, aligning with our simulation results (Supplementary Fig. 11).
In vdW metal sulfides, Vs are the most prevalent defects, acting as active sites for the insertion and removal of metal ions, which facilitates resistive switching and has been widely studied38,39,40. In contrast, VZn dominate in our ZnPS3 materials, yet the role of metal vacancies in vdW materials in the formation of metallic conductive filaments remains poorly understood. Therefore, we focus on the influence of VZn on Ag ion migration. We compared the diffusion energy barrier for Ag migration in both pristine (without VZn) and zinc-deficient ZnPS3 using a three-layer mode. Ag migration within ZnPS3 begins with bonding to sulfur atoms and then proceeds into the gaps between zinc atoms. The high flexibility of the [P2S6]4− polyanions allows Ag ions to migrate across the ZnPS3 layer by altering P-P and P-S bond lengths, as well as S-P-P bond angles, as shown in the insert of Fig. 2e and Supplementary Fig. 12a. The calculated diffusion energy barrier for Ag ion migration through the first layer of pristine ZnPS3 is 0.90 eV. However, when a VZn is introduced in the first layer of ZnPS3, Ag ions can directly hop into the VZn site (Fig. 2f, inset, and Supplementary Fig. 12b), reducing the diffusion energy barrier to 0.62 eV, indicating that VZn significantly facilitates Ag ion migration. The diffusion energy barriers for Ag ion migration in the second layer remain almost unchanged, with values of 1.82 eV for pristine ZnPS3 and 1.80 eV for Zn-deficient ZnPS3, suggesting that VZn in the first layer has minimal effect on the diffusion barrier in subsequent layers. XPS analysis estimates the concentration of VZn in ZnPS3 to be around 10%, implying a high density of VZn in the material. As suggested by statistical thermodynamics, pathways with lower energy barriers are much easier to overcome than those with higher barriers24. Therefore, Ag ions tend to migrate along the VZn, which considerably lowers the overall diffusion energy barrier for Ag migration across multilayer ZnPS3. This reduction in the diffusion energy barrier leads to lower energy consumption for resistive switching, making ZnPS3 memristors highly suitable for ultralow energy neuromorphic computing applications. Detailed dynamic processes of Ag ion migration in Zn-defective ZnPS3 are shown in Fig. 2g, h, while those in pristine ZnPS3 are provided in Supplementary Figs. 13 and 14.
Volatile resistive switching for use in artificial neuron
As exhibited in Fig. 3a, a typical biological neuron consists of a soma, dendrites, and axons, which collectively enable it to integrate input signals from presynaptic neurons in a spatiotemporal manner and transmit these signals to postsynaptic neurons through the firing of action potentials. Volatile memristors, capable of nonlinearly transforming input electrical pulse signals and exhibiting temporal dynamics of higher-order complexity, are promising candidates for mimicking the functions of artificial neurons. Figure 3b presents the I-V characteristics of our ZnPS3 memristor, demonstrating stable unipolar volatile resistive switching during periodic voltage sweeps at a low compliance current of 1 μA. The high off-state resistance (~1012 Ω) effectively reduces static power consumption and crosstalk. Statistical analysis of the I-V curves in Fig. 3b reveals that the average threshold voltage Vth (Fig. 3c) and hold voltage Vhold (Fig. 3d) of the ZnPS3 memristor are approximately 0.180 V and 0.058 V, respectively, which are comparable to most of the best-performing volatile memristors (Supplementary Table 2 and Supplementary Fig. 15). Furthermore, the memristor demonstrates exceptional endurance, sustaining over 106 switching cycles under repeated positive pulse stimulation while maintaining a high on/off current ratio of approximately 104 with minimal performance degradation, as shown in Fig. 3e. During prolonged cycling, successive positive pulses gradually displace Ag atoms from their source electrode toward the opposing electrode, thereby depleting the reservoir of mobile Ag and reducing the availability of atoms for filament formation. This progressive depletion can lead to a decline in low-resistance state (LRS) conductance at even higher switching cycles41. Importantly, this degradation is reversible: applying a negative bias effectively drives Ag atoms back to their original electrode, restoring the LRS conductance and device performance.
Fig. 3: Volatile switching for mimicking artificial neuron.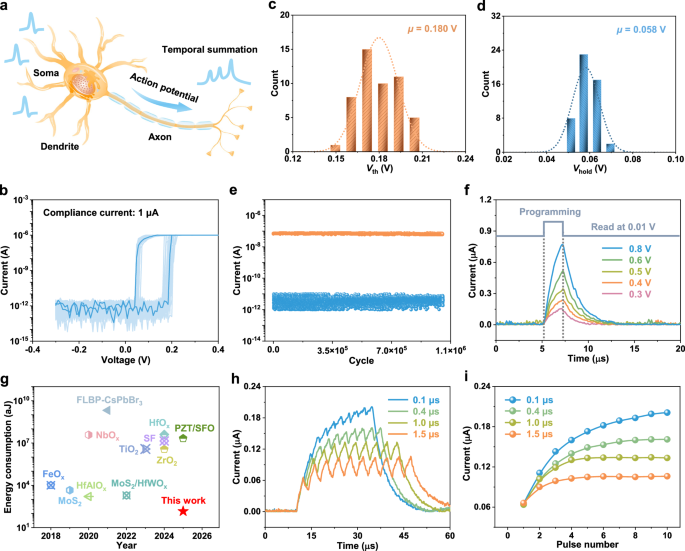
a Schematic diagram depicting a biological neuron. b I-V curves of the ZnPS3 memristor for 50 consecutive sweeps. c, d Statistical analysis of threshold voltage Vth (c) and hold voltage Vhold (d) of the ZnPS3 memristor, where μ stands for the mean value. e Endurance of the device for 106 switching cycles. f Current evolution of the ZnPS3 memristor during electrical pulse stimulation with different amplitudes and during subsequent readout at 0.01 V. g Comparison of volatile memristive switching based on various switching layers, demonstrating the low energy consumption of the ZnPS3 memristor. Detailed references are provided in Supplementary Table 3. h Time-dependent current of the ZnPS3 memristor stimulated by pulse train with different intervals. i Current as a function of pulse number.
The volatile switching behavior is further demonstrated using electrical pulse stimuli. As shown in Fig. 3f, the current through the memristor increases nonlinearly and continuously during pulse stimuli, indicating effective programming. Following excitation, a gradual current decay is observed under a continuously applied read voltage of 0.01 V, exhibiting the characteristics of volatile switching. This read voltage is well below both the threshold and hold voltages of the ZnPS3 memristor, and therefore does not induce further resistive switching. Moreover, pulses with high amplitude lead to extended relaxation times (Supplementary Fig. 16), indicating desirable dynamics for mimicking biological processes. The highly reproducible, time-dependent current highlights the reliability of the device, as shown in Supplementary Fig. 17. The minimal energy consumption of the ZnPS3 memristor operating in the volatile switching mode was evaluated using a 10-ns electrical pulse with an amplitude of 0.7 V, yielding an ultralow switching energy of approximately 143 aJ per operation (Supplementary Fig. 18). This performance surpasses that of previously reported volatile memristors, as summarized in Fig. 3g and Supplementary Table 3. This energy consumption can be further reduced to approximately 63 aJ by using weaker pulses, as shown in Supplementary Fig. 19. It is important to clarify that the energy consumption reported here pertains solely to the energy required for initiating volatile switching, corresponding to the 10 ns programming pulse. The subsequent current decay observed under the applied read voltage reflects a passive relaxation process, attributed to the spontaneous dissolution of thin Ag conductive filaments41, and thus the energy dissipated during this period does not contribute to switching. In neuromorphic applications such as reservoir computing, the device current is commonly sampled during the programming pulse itself, without the application of an additional read voltage42. Accordingly, focusing the energy analysis on the programming pulse alone is consistent with established methodologies17,42.
Fading memory is a vital feature for artificial neuron, enabling them to learn and process information in dynamic environments. Here, pulses with different intervals are applied to the ZnPS3 memristor to demonstrate its fading memory. As exhibited in Fig. 3h, i, when the temporal offset (pulse interval) is larger than the relaxation time, the conductance always returns to the same state. On the contrary, different responses are achieved if the pulse interval is smaller than the relaxation time, where a continuous increase in conductance is observed. The demonstrated nonlinearity and fading (short-term) response of the ZnPS3 memristor enable it to project the input signals into a new domain, facilitating linear classification, which is well-suited for information processing and encoding.
Non-volatile resistive switching characteristics for artificial synapse
A biological synapse, located at the junction between a pre-neuron and a post-neuron, facilitates signal transmission through the release of neurotransmitters, as shown in Fig. 4a. The synaptic weight, or strength of synaptic connections, can be modulated by varying the stimulus conditions. Similarly, in our ZnPS3 memristor, non-volatile resistive switching is achieved by forming thick Ag filaments using a higher compliance current or even a single stronger electrical pulse, enabling them to function as an artificial synapse capable of storing multiple synaptic weight values.
Fig. 4: Non-volatile switching for artificial synapse.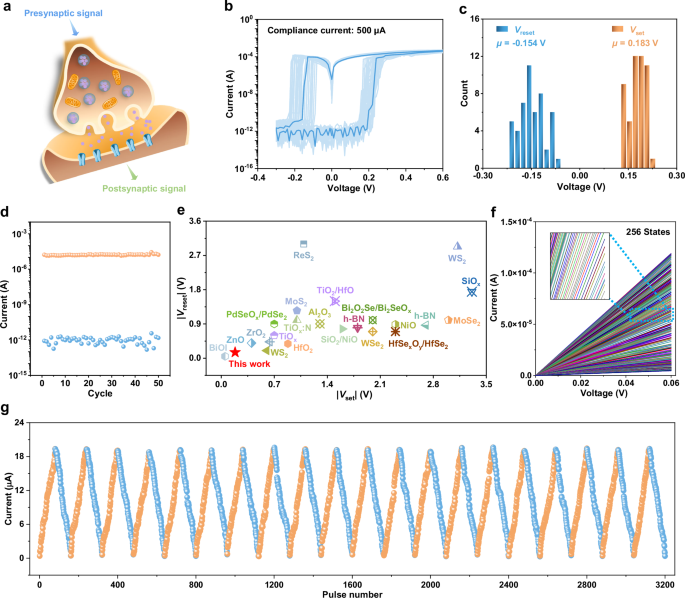
a Schematic diagram exhibiting a biological synapse. b I-V curves of the ZnPS3 memristor for 50 consecutive sweeps. c, d Statistical analysis of the set voltage Vset and reset voltage Vreset (c), and on/off ratio (d). e Comparison of the operating voltages of the ZnPS3 memristor in non-volatile switching mode with those of non-volatile memristors based on other two-dimensional materials and oxides, indicating the low switching voltages of the ZnPS3 memristor. Detailed references are provided in Supplementary Table 4. f Multi-level conductence. g Long-term plasticity of LTP and LTD stimulated by pulse train with varying pulse amplitude for 20 consecutive cycles.
Figure 4b exhibits the cyclic I-V characteristics of the ZnPS3 memristor at a high compliance current of 500 μA, demonstrating repeatable non-volatile bipolar resistive switching behavior. Statistical analysis of the operation voltage, extracted from the I-V curves in Fig. 4b, reveals average Vset and Vreset of approximately 0.183 V and −0.154 V, respectively, for the non-volatile switching mode (Fig. 4c). The device also exhibits a significant switching ratio of approximately 107, as shown in Fig. 4d. Both Vset and Vreset of the ZnPS3 memristor are among the lowest reported for non-volatile memristive devices, as shown in Fig. 4e and Supplementary Table 4. The energy required for non-volatile switching was assessed using a 20-ns electrical pulse at an amplitude of 1.15 V, resulting in a remarkably low switching energy of approximately 28 fJ (Supplementary Fig. 20). This value places the device among the most energy-efficient non-volatile memristors reported to date, as summarized in Supplementary Fig. 21 and Supplementary Table 5. Furthermore, as shown in Fig. 4f, the ZnPS3 memristor demonstrates a wide range of conductive states, with at least 256 distinct levels (8 bits), making it highly suitable for synaptic weight storage. The I-V characteristics of the ZnPS3 memristor across different conductance states show excellent linearity, facilitating accurate linear dot product operations for ANN-based neuromorphic computing tasks. The ZnPS3 memristor demonstrates stable retention across multiple conductance states for up to 3.6 × 10⁴ s, with minimal degradation observed over this duration, as shown in Supplementary Fig. 22. This retention performance is comparable to that of recently reported artificial synaptic devices developed for neuromorphic computing, as summarized in Supplementary Table 6. It is adequate for short-term inference and classification tasks typically encountered in neuromorphic applications, such as ECG classification, chaotic signal prediction, gesture recognition, voice command recognition, and electroencephalogram analysis. For use cases that demand longer retention times, additional strategies such as refresh schemes or hybrid memory architectures may be employed. In refresh schemes, periodic reinforcement pulses can be applied to sustain conductance levels prior to noticeable relaxation. In hybrid memory configurations, long-term storage elements such as flash memory can be used to retain trained synaptic weights, which can then be selectively reloaded into the ZnPS3 memristors when required. These approaches enable practical utilization of the characteristics of the ZnPS3 memristor such as its reconfigurability, low operating voltage, and low switching energy in adaptive neuromorphic systems.
Analogue modulation of conductance states is crucial for the high accuracy required in ANNs. In Ag filament-based memristors, the switching characteristics are highly influenced by the measurement methodology, which can determine whether the device exhibits abrupt digital-like transitions or gradual analog modulation of conductance43,44,45. Under DC I–V measurements, the relatively slow voltage sweep leads to a prolonged electric field application at each voltage step, facilitating the rapid formation or rupture of Ag conductive filaments. This results in sharp set and reset transitions, as illustrated in Fig. 4b and Supplementary Fig. 23. In contrast, the application of carefully tailored pulse train schemes enables a more controlled, stepwise modulation of the conductive filaments. This allows for the realization of gradual conductance tuning, as shown in Supplementary Fig. 24. When using a pulse train consisting of 80 consecutive positive pulses followed by 80 consecutive negative pulses, each with varying amplitudes, conductance tunability with improved linearity and symmetry can be achieved, as shown in Fig. 4g and Supplementary Fig. 25. During modulation, the current increases as the number of positive pulses increases and decreases with the application of negative pulses, mimicking the behavior of LTP and LTD, which are associated with the strengthening and weakening of synaptic connections, respectively. Supplementary Fig. 26 presents the conductance modulation characteristics of 25 individual ZnPS3 memristors, indicating device-to-device variation with a mean-to-standard deviation ratio below 0.3 across all conductance states. This variability may be further reduced by refining the synthesis of ZnPS3 single crystals to achieve a more uniform distribution of VZn, as well as by introducing pre-deposited, uniformly distributed Ag nanocluster seeds to guide filament formation37,41. The endurance of the device is approximately 104 switching cycles, as shown in Supplementary Fig. 27, which is comparable to previously reported vdW memristors22,46. Endurance may be further enhanced by optimizing the pulse programming scheme or by incorporating external compliance resistors to mitigate excessive filament growth.
Reservoir computing system simulation
Reservoir computing has been introduced as an alternative to conventional recurrent neural networks by utilizing relatively fixed, nonlinear reservoirs to process temporal data47,48. A typical reservoir computing framework comprises a volatile reservoir that transforms input signals into a high-dimensional dynamic state space, and a non-volatile readout layer that interprets these states49. The nonlinear transformation of the reservoir facilitates the conversion of complex inputs into a form that can be separated using linear methods, allowing the readout layer to be trained using simple techniques such as linear regression or back-propagation. This architecture simplifies the training process, reduces computational overhead, and enables rapid learning with limited training samples50,51. Additionally, the ability of reservoirs to encode features over multiple timescales makes reservoir computing particularly suitable for analyzing temporal sequences and forecasting time-series data47. Applications of reservoir computing span various domains, including dynamical system modeling, speech processing, physiological signal classification, chaotic behavior prediction, financial analysis, and hydrological forecasting42,52,53,54. Fully memristive reservoir computing systems, which utilize volatile memristors in reservoir layers and non-volatile memristors in readout layers, are emerging as compact, energy-efficient neuromorphic hardware with high efficacy in processing and classifying spatiotemporal signals1,55,56. Our ZnPS3 memristors, exhibiting intrinsic dynamic properties, nonlinear current responses, fading memory, and echo state characteristics in volatile mode, are well-suited as dynamic memristor node for deployment in reservoir layers. Additionally, the analogue modulation of LTP/LTD and stable long-term memory retention in the non-volatile mode make ZnPS3 memristors ideal for emulating ANNs in readout layers. The low switching voltages and energy consumption of ZnPS3 memristors surpass those of previously reported advanced reconfigurable memristors (Supplementary Fig. 28), positioning them as promising candidates for ultralow energy adaptive neuromorphic computing.
To demonstrate the practical utility of ZnPS3 memristors, we simulate a reservoir computing system that incorporates a volatile virtual node reservoir computing layer alongside an ANNs readout layer with non-volatile weights to perform arrhythmic heartbeat classification, a representative benchmark temporal task (Fig. 5a). For the ECG classification task, we employed the MIT-BIH Arrhythmia Database, a widely used benchmark dataset that comprises 48 half-hour, two-channel ECG recordings sampled at 360 Hz from 48 individual subjects57,58. The dataset contains a diverse range of both normal and abnormal heartbeat patterns. In accordance with the classification scheme defined by the Association for the Advancement of Medical Instrumentation, the heartbeats are grouped into five categories (Supplementary Table 7): normal (N), fusion (F), supraventricular ectopic (S), ventricular bigeminy (Q), and premature ventricular contraction (V)59. For this study, a total of 1000 heartbeats were randomly selected from the dataset, comprising 200 samples from each class. These were randomly partitioned into training and testing sets in a 70:30 ratio, maintaining class balance. Specifically, 700 heartbeats (140 per class) were used for training, while the remaining 300 heartbeats (60 per class) were used for model evaluation.
Fig. 5: Reservoir computing system simulation.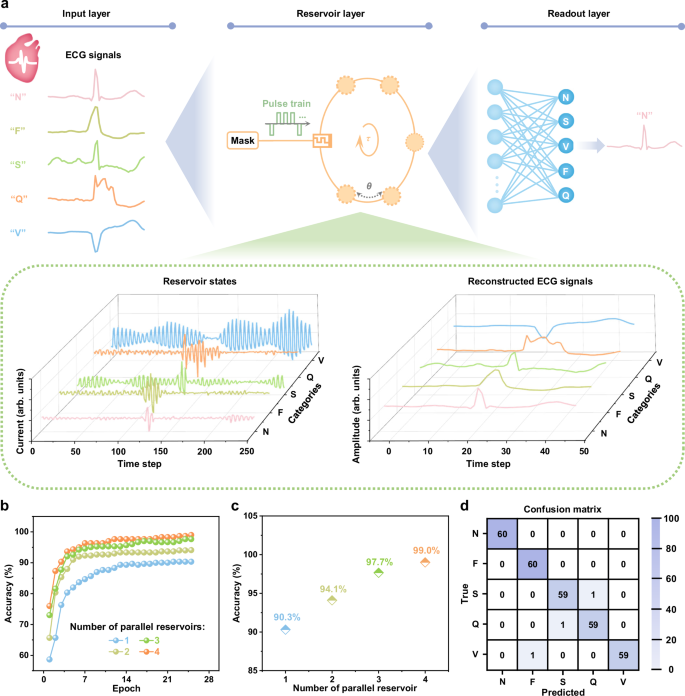
a Schematic diagram of the reservoir computing system using the reconfigurable ZnPS3 memristors as both reservoirs and readout layer. Output current signals of the ZnPS3 memristor generated by feeding electrocardiogram (ECG)-converted pulse train, as well as reconstructed ECG signals. b Classification accuracy as a function of epoch of 4 individual reservoirs. c Classification accuracy as a function of paralleled reservoir number. d Confusion map of the reservoir system based on 4 paralleled reservoirs.
In the reservoir layer, a time-multiplexing strategy was implemented by applying a masking sequence to the ZnPS3 memristor, enabling the formation of time-domain virtual nodes and generating diverse reservoir states. The volatile switching behavior of the ZnPS3 memristor, characterized by nonlinear response and fading memory, allows it to function as a single nonlinear node with delayed feedback, thereby providing a compact and resource-efficient platform for reservoir computing. Specifically, the masking sequence of length N divides the delay period τ into N equidistant intervals, with each interval defined as θ = τ/N. These N time segments correspond to virtual nodes, whose states are determined by the current response of the memristor. To establish temporal interactions among the virtual nodes, θ was set to be shorter than the characteristic relaxation time of the memristor, thereby approximating recurrent dynamics within the reservoir.
Before conducting the ECG classification task, a preliminary waveform classification test was performed to assess the temporal signal processing capability of the ZnPS3-based reservoir. This task involved distinguishing between randomly generated sine and square waveforms (Supplementary Fig. 29a). The classification performance was evaluated using the normalized root mean squared error (NRMSE) across different configurations of parallel reservoirs and virtual nodes, as shown in Supplementary Fig. 29b. A limited number of reservoirs or virtual nodes led to diminished performance due to insufficient diversity in the reservoir states. Conversely, increasing the number of reservoirs and virtual nodes beyond a certain point only marginally improved the NRMSE while introducing redundant states that could lead to higher energy consumption, increased readout layer complexity, and greater circuit overhead. An optimal configuration comprising four parallel reservoirs and five virtual nodes yielded low NRMSE and reliable classification performance (Supplementary Fig. 29c), representing a practical trade-off among accuracy, computational cost, and system complexity. This configuration was subsequently employed for the ECG classification task. To form the delay loop for ECG signal processing, each data point in the input ECG signal sequence was multiplied by a one-dimensional vector containing five randomly assigned binary values (−1 and 1), and then converted into a 5-timeframe pulse train containing only write pulses. This process resulted in the creation of 5 virtual nodes per memristor for each input data point, with the current responses representing the reservoir states of these virtual nodes. Figure 5a displays typical reservoir states of various ECG signals from the ZnPS3 memristors, which were used to accurately reconstruct the ECG signals.
In the readout layer, a single-layer ANN was implemented, comprising 250 input neurons, 5 output neurons, and 250 × 5 synaptic connections, as illustrated in Supplementary Fig. 30. The synaptic weights Wi,j, where i and j represent the indices of the input and output neurons respectively, were encoded by the conductance states of ZnPS3 memristors. The training and testing procedures of the ANN are outlined in Supplementary Fig. 31. During training, the reservoir states derived from the ECG training dataset were applied to the input neurons, and the resulting signals were weighted by the corresponding memristive synapses and summed at the output neurons to generate output currents Ij. These currents were passed through a Softmax activation function to produce the output probabilities Yj, which were then compared to the ground-truth labels. The error between the predicted outputs and true labels was used to adjust the synaptic weights via a backpropagation algorithm, informed by the LTP/LTD characteristics of the device. Details of the weight update mechanism are provided in the “Methods” section. During testing, the trained weight matrix was applied to the reservoir states of the test dataset to generate predictions, which were compared against the actual labels to assess classification performance. A conceptual circuit-level implementation of this reservoir computing system is presented in Supplementary Figs. 32–35.
Figure 5b illustrates the classification accuracy of the experimentally obtained results with varying numbers of individual ZnPS3 memristors. Classification accuracy improved with an increasing number of epochs and reservoir elements. As shown in Fig. 5c, by parallelizing four ZnPS3 memristor-based reservoirs, the system achieved enhanced accuracy in distinguishing regular heartbeats. An overall classification accuracy of 99% was achieved across all categories, with accuracy for individual class recognition reaching at least 98.3% (Fig. 5d). As summarized in Supplementary Table 8, the ECG classification accuracy achieved using the ZnPS3 memristor-based reservoir computing system is comparable to those reported in recent studies on state-of-the-art neuromorphic platforms. Beyond classification of complex biomedical waveforms such as ECG signals, the system also exhibits effective performance in chaotic time-series prediction. To evaluate this capability, a benchmark task involving the Hénon map—a well-known discrete-time chaotic system—was carried out. As shown in Supplementary Fig. 36a, the predicted outputs closely follow the target values after training, indicating accurate temporal prediction. A two-dimensional representation of the Hénon map is provided in Supplementary Fig. 36b, further illustrating the agreement between the predicted and actual trajectories.
The proposed neuromorphic hardware, combining volatile memristors in reservoir layers and non-volatile memristors in readout layers, has the potential to revolutionize applications requiring efficient spatiotemporal data processing. Its compact form factor and low energy consumption make it particularly well-suited for edge computing, where accurate analysis of time-series data is critical. This is especially pertinent in healthcare monitoring, where the human body generates vast and complex data streams during daily activities. Efficient processing and classification of this data are vital for timely disease detection and effective health management, reducing the strain on medical resources and enhancing quality of life. In particular, our device has demonstrated remarkable performance in processing and classifying ECG signals, showing significant potential for arrhythmia detection. Beyond ECG analysis, the device is adaptable to a broad spectrum of health-monitoring applications, including electroencephalograms, blood oxygen saturation, blood pressure, glucose levels, respiratory conditions, motion and gait analysis, speech and swallowing patterns, and body temperature. By leveraging reconfigurable ZnPS3 memristors, this ultralow-energy neuromorphic hardware offers a powerful, compact, and energy-efficient platform for advancing healthcare monitoring and other time-series data processing tasks.