Cell response to matrix viscoelasticity is stiffness- and Piezo1-dependent
Two different hydrogel pairs were synthesised by mixing different amounts of acrylamide (AAm) and bis-acrylamide (BisAam) to obtain hydrogels of approximately the same E but with varying stress relaxation rates. To achieve soft (E ≈ 0.4 kPa) and stiff (E ≈ 25 kPa) hydrogels, we combined two previously reported strategies to tune viscoelasticity in PAAm hydrogels (Fig. 1a).
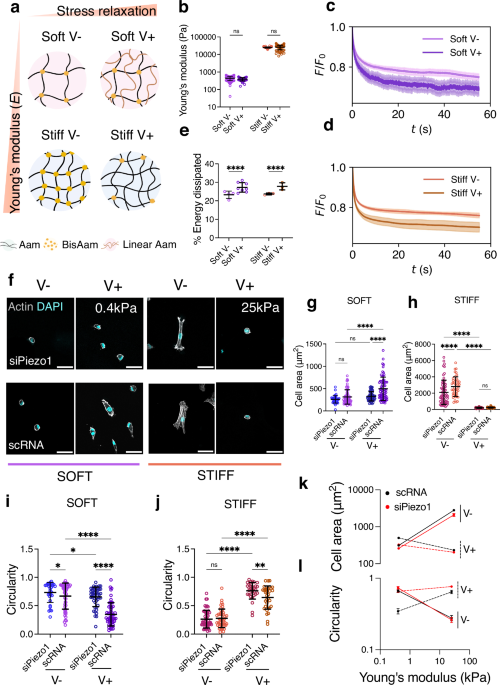
Fig. 1: Cell response to matrix viscoelasticity is stiffness and Piezo1 dependent.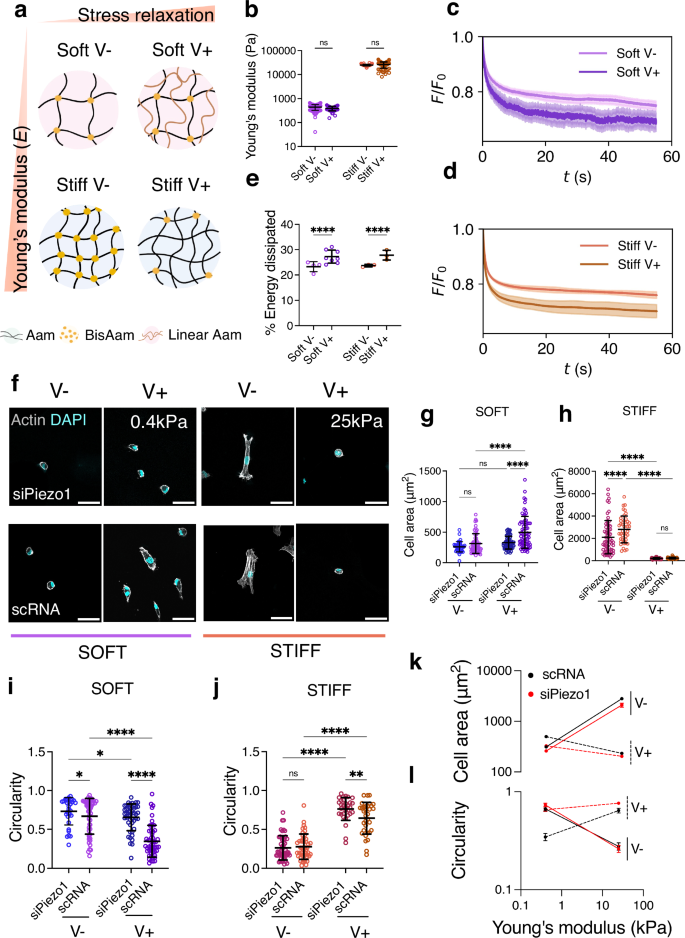
a Representation of the different hydrogel networks. Here and in the other figure panels, V− represents an elastic, slow-relaxing hydrogel and V+, a fast-relaxing, viscoelastic hydrogel. Aam: Acrylamide, BisAam: Bisacrylamide, Linear Aam: Linear Acrylamide. Gel network diagram was created with BioRender b Nanoindentation measurements, showing Young’s modulus data obtained for Soft (0.4 kPa, purple) and Stiff (25 kPa, orange) hydrogel pairs. Data shown as individual values, mean ± SD (from left to right n = 95, 58, 113, 80 single indentation curves, from N ≥ 3 gels). c Representative average stress relaxation curve ± SD of an indentation performed on soft (purple) and d stiff (orange) hydrogels. e Average energy dissipated in soft (purple) and stiff (orange) hydrogel groups, shown as individual values, mean ± SD. From left to right n = 4, 8, 3 and 3 indentations maps, comprising 61, 87, 41 and 71 total indentations on N = 3 hydrogels. f Representative Actin and DAPI immunofluorescence images of control (scRNA) and Piezo1 knock down (siPiezo1) Y201 MSCs cultured on different hydrogel groups for 48 h. Scale bar = 50 µm g, h Quantified cellular area on the soft (left) and stiff (right) hydrogel groups. All individual points represent individual cell measurements. Data shown as individual values, mean ± SD, in (g) from left to right n = 47, 58, 59 and 66 cells; in (h) from left to right n = 62, 42, 40 and 33 cells. Data from N = 3 independent experiments. i, j Quantified cellular circularity of the soft (left) and stiff (right) hydrogel groups. Data shown as individual values, mean ± SD. In (i) from left to right n = 32, 50, 47 and 51 cells; in (j) from left to right n = b 52, 46, 31 and 33 cells. Data from N = 3 independent experiments. k Summary of mean cellular area ± SEM plotted as a function of stiffness for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions. l Summary of mean cellular circularity ± SEM plotted as a function of stiffness for for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions. Statistical analyses were performed using a two-way ANOVA test. P values indicating significance, ns > 0.05, *≤0.05, **≤0.01, ***≤0.001, ****≤0.000. Specific p values and descriptive statistics are provided in the Source Data.
To generate the stiff hydrogel pair, we used the method first reported by Cameron and colleagues23,24 and later optimised by our group25, in which substrate viscoelasticity is mediated by the movement of loosely crosslinked polymer chains. To create a softer pair of viscoelastic gels, we used the approach reported by Charrier and colleagues26,27, as the Cameron et al. approach, when modified to create softer hydrogels, gave rise to sticky hydrogels that were difficult to handle. In the Charrier et al. method, substrate viscoelasticity arises from physically entrapped chains of high molecular weight, linear Aam (Fig. 1a). The resulting hydrogel groups had a Young’s modulus of approximately 0.4 kPa and 25 kPa (hereafter referred to as soft and stiff, respectively); we observed no significant differences in Young’s modulus within each stiffness group (Fig. 1b). To characterise the differences in the hydrogel’s stress relaxation behaviour, we performed stress relaxation measurements with a physiologically relevant step strain (ε) of 7%28,29 applied over 60 s using nanoindentation. From the resulting stress relaxation curves (Fig. 1c, d), we calculated the time for the stress to relax to 80% of the initial value (Supplementary Data Fig. 1a), as well as the % of energy dissipation for each hydrogel condition (Fig. 1e). Resulting data demonstrated that for each stiffness group, there was a slow-relaxing (V-, elastic) and fast-relaxing (V+, viscoelastic) hydrogel, and that the V+ hydrogels relax ∼2 times faster than their elastic counterparts (Fig. 1c, d), and display higher relaxation amplitude, shown as % of energy dissipated (Fig. 1e). To confirm data obtained by nanoindentation, we additionally performed shear bulk rheology measurements. By computing the ratio between the loss modulus (G”) and the storage modulus (G’), we observed that the tan (δ) of the V+ hydrogels increased for both stiffness groups with respect to V- hydrogels (Supplementary Data Fig. 1b, c), emulating soft tissues that exhibit loss moduli of approximately 10% of their elastic moduli at 1Hz8. Notably, as PAAm hydrogels are chemically crosslinked, both strategies give rise to viscoelastic solids with no plastic deformation, in contrast to physically crosslinked viscoelastic hydrogels30. These properties allowed us to investigate the cell response to substrate viscoelasticity independently of substrate elasticity, or Young’s modulus, in two distinct stiffness regimes in 2D. Moreover, we performed nanoindentation curve contact point analysis to rule out any potential differences in hydrogels’ topography due to i) the presence of linear acrylamide in the soft group and ii) the different ratios of monomer and crosslinker in the stiff group (Supplementary Data Fig. 1f, g). Next, we checked that the ECM matrix protein FN, the ECM element of the molecular clutch, was homogeneous on all substrates regardless of their stiffness and viscoelasticity, as previously reported23,26. By using the crosslinker sulfo-SANPAH, FN was conjugated on each hydrogel31. By using immunofluorescence, we confirmed a homogenous FN coating on all substrates with no significant changes in signal intensity (Supplementary Data Fig. 1d, e). Overall, these findings show that we have established a hydrogel system that spans a wide range of physiologically relevant stiffnesses with different rates of stress relaxation, allowing for the investigation of the effects of ECM viscoelasticity on cell behaviour independently of ligand density and topography.
We next investigated Piezo1 gene expression of control Y201 MSCs (scRNA) and of MSCs in which Piezo1 was knocked down via small interfering RNA (siRNA) for Piezo1 using an siRNA screen (siRNAs 1–3) (Supplementary Data, Fig. 2a). Additionally, we assessed morphology and adhesions on all screened conditions plus on cells treated with the mechanosensitive ion channel inhibitor GsMTx4 (Supplementary Data, Fig. 2b, c). This allowed us to select the most consistent siRNA, siRNA1, which was used subsequently in all experiments and referred to as siPiezo1. siPiezo1-mediated Piezo1 knock down demonstrated clear phenotypic differences in cell morphology and adhesion formation (Supplementary Data, Fig. 2d), as well as approximately 50% efficiency in Y201 MSCs in terms of protein and gene expression levels (Supplementary Data, Fig. 2e, f). Following this, we assessed the morphology of scRNA and siPiezo1 MSCs on all hydrogel conditions (representative images in Fig. 1f). On soft hydrogels, scRNA MSCs increased their spreading area in response to substrate stress relaxation, whereas on stiff substrates, the opposite occurred (Fig. 1g, h). This response was also reflected in terms of their circularity (Fig. 1i, j). These results corroborate data first reported by Chaudhuri and colleagues in cancer epithelial cells9, which indicated that cell spreading in response to increased substrate relaxation is stiffness-dependent and only increases at lower stiffness values in stress-relaxing substrates (22).
In terms of Piezo1-dependent morphology, knock down has previously been reported to disrupt actin fibres and integrin activity, thus decreasing cell spreading32. In this study, siPiezo1 MSCs decreased their spreading area and increased their circularity, as compared to scRNA MSCs, when cultured on soft viscoelastic (V+) or stiff elastic (V-) hydrogels (Fig. 1k, l). Interestingly, the cell-spreading response to viscoelasticity seen in scRNA MSCs was abrogated in siPiezo1 MSCs cultured on low stiffness (soft, 0.4 kPa) but not on stiff substrates (stiff, 25 kPa), as these cells had presumably reached their minimum spreading area independently of Piezo1. This Piezo1-mediated cell spreading behaviour was also observed when we cultured MSCs on FN-coated glass substrates (Supplementary Data, Fig. 2d, e, f), where siPiezo1 cells showed reduced cell spreading area compared to scRNA MSCs. These data endorse the overall suppressive effect of channel knock down on cell-substrate interaction, as previously reported in other cell types13. Together, our results identify a Piezo1-dependent response to matrix viscoelasticity in soft matrices.
Molecular clutch engagement in response to matrix viscoelasticity is Piezo1-dependent
To further investigate the mechanism of Piezo1-mediated ECM viscoelasticity sensing, we quantified the engagement of the actin-talin-integrin-FN molecular clutch in MSCs cultured on the different substrates in the presence and knock-down of Piezo1. Clutch dynamics regulate cell mechanotransduction in response to substrate stiffness6, viscosity7 and more recently, viscoelasticity9,22. However, how and whether Piezo1 relays ECM viscoelasticity cues via the molecular clutch is unknown. We first quantified FA formation by looking at vinculin using immunofluorescence, as vinculin is recruited to adhesion sites in response to sustained force-sensing by the actin-talin-integrin-FN clutch6 (Fig. 2a). Individual vinculin FA length (Fig. 2b, c) and FA count (Supplementary Data Fig. 3a–c) were quantified in siPiezo1 and scRNA MSCs cultured on the soft and stiff hydrogel groups. The resulting data supported the previously observed cell-spreading phenotype: FA length increased in scRNA MSCs under conditions of faster substrate stress relaxation in a soft regime (0.4 kPa), while FA length decreased in scRNA MSCs cultured on stiff (25 kPa) hydrogels in response to enhanced stress relaxation (Fig. 2d).
Fig. 2: Focal adhesion formation in response to matrix viscoelasticity is stiffness and Piezo1-dependent.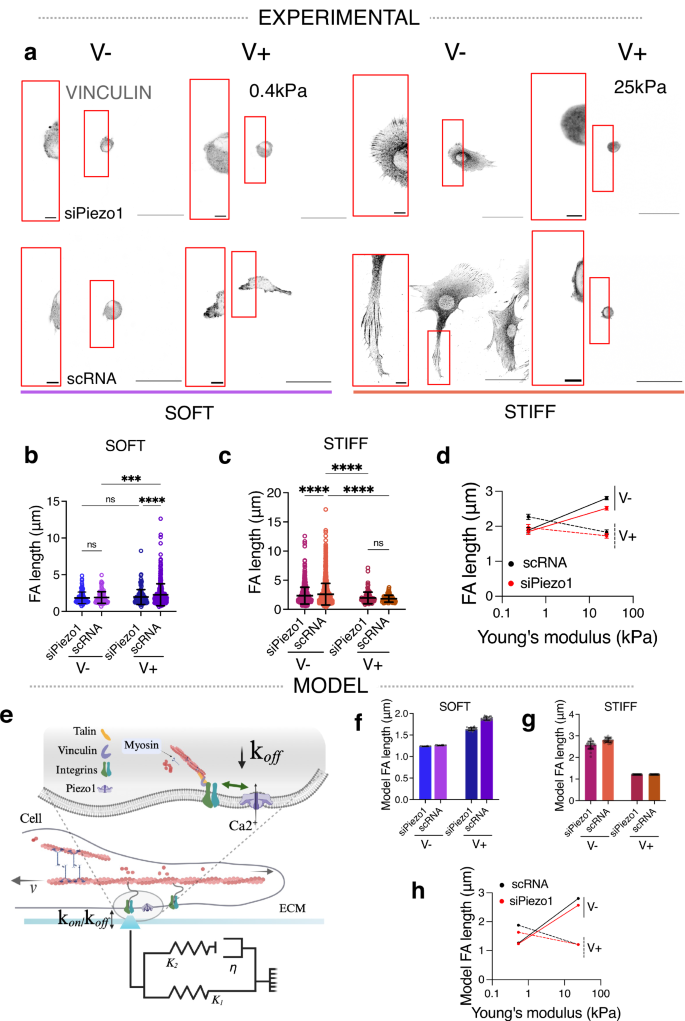
a Representative images of immunostained vinculin adhesions in siPiezo1 and scRNA Y201 MSCs cultured on soft (0.4 kPa, left) and stiff (25 kPa, right) hydrogel groups of varying stress relaxation rates. Scale bar = 50 µm, zoomed image scale bar = 5 µm. b, c Quantified individual focal adhesion (FA) length of cells on the soft (left) and stiff (right) hydrogel groups. In (b) from left to right n = 103, 104, 130 and 371 individual FA measurements from 30, 31, 32 and 39 cells; in (c) from left to right n = 689, 1289, 103 and 76 individual FA measurements from 30, 31, 31 and 35 cells. Data from N = 3 independent experiments. Data shown as individual values, mean ± SD. d Summary of mean individual FA length ± SEM plotted as a function of stiffness for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions. Statistical analyses were performed using a two-way ANOVA test. P values indicating significance, ns > 0.05, *≤0.05, ***≤0.001, ****≤0.0001. Specific p values and descriptive statistics are provided in the Source Data. e Schematic of the influence of Piezo1 in molecular clutch engagement. Cell is shown to be coupled to the ECM via ECM-binding integrins that in turn, connect to the contractile actin filaments via mechanosensitive adaptive proteins (talin and vinculin). Myosin motors continuously pull on actin filaments with velocity (v). Here, the ECM is modelled as a Standard Linear Solid (SLS), composed of two elastic springs (K1 and K2) and a viscous dashpot element (η). kon and koff represent the rates of clutch association and dissociation, respectively. In the zoom (grey shading), the concerted action between Piezo1 and integrins is highlighted, showing that potentiation of this interaction decreases clutch dissociation (koff), created with BioRender. f, g Scaled model predictions of focal adhesion length (µm) on the soft (left) and stiff (right) substrate groups. Data shown as individual values, mean ± SD (N = 26 simulations). h Summary of model predictions for mean scaled focal adhesion length (µm) ± SEM plotted as a function of substrate stiffness for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions.
Since Piezo1 was first identified in 2010 as an integrin co-activator, its activity has been intrinsically linked to FA dynamics13,14,15,16,17,32. Indeed, Piezo1 knock down was sufficient to visibly reduce FA size and number in Y201 MSCs cultured on FN-coated glass substrates (Supplementary Data Fig. 2e). Unlike scRNA MSCs, siPiezo1 MSCs did not exhibit increased FA size (Fig. 2b) or number (Supplementary Data Fig. 3a) when seeded on soft, fast-relaxing matrices (soft V+) as compared to elastic (soft V−) substrates. This highlights the fundamental role of this channel in mediating cell response to matrix viscoelasticity at low substrate stiffness. Both siPiezo1 and scRNA MSCs exhibited significantly decreased FA length (Fig. 2c) and number (Supplementary Data Fig. 3b) when cultured on stiff V+ substrates, compared to stiff V-. However, the magnitude of this change was lessened in siPiezo1 MSCs. Similarly, when culturing scRNA and siPiezo1 MSCs on FN coated glass substrates, siPiezo1 MSCs had fewer FA structures compared to scRNA MSCs (Supplementary Data Fig. 2f). These results further established that Piezo1 channel activity is inherently linked to FA dynamics.
We then tested whether a computational model of the molecular clutch could capture both the effect of stiffness in mediating cell response to viscoelasticity, as previously reported9,22 and as seen experimentally in this work; and the Piezo1-dependent clutch engagement on soft matrices. The model, based on our previous work6, simulates the stochastic cell adhesion dynamics on a viscoelastic substrate using a Monte Carlo approach that captures integrin-ligand binding, force transmission and adhesion growth (Fig. 2e). To model the response to a viscoelastic substrate, we modified our previous model to consider a viscoelastic rather than elastic substrate, modelled as a Standard Linear Solid (SLS). The SLS exhibits instantaneous resistance to deformation and time-dependent relaxation, as observed experimentally (see Fig. 1c, d), for details see Supplementary Note 1. Upon integrin binding to the substrate-bound ligands, myosin-generated forces build up in the clutch, leading to talin unfolding, vinculin binding and subsequent adhesion growth (modelled as integrin recruitment). Taking the soft elastic condition as reference, increasing stiffness (soft V− vs stiff V− conditions) results in higher forces and hence greater FAs in experiment (Fig. 2d) and theory (Fig. 2h). Increasing viscosity (soft V- versus soft V+ conditions) also leads to greater adhesion growth in experiment (Fig. 2b) and theory (Fig. 2f). This can be explained from both additional forces due to viscous resistance, and the effect of time-varying elasticity and hence force, affecting clutch buildup and lifetime22. However, within the viscoelastic conditions, increasing stiffness (soft V+ versus stiff V+ conditions) reduces adhesion length (Fig. 2d), and thus integrin recruitment. To explain this in the model, this requires considering that integrin recruitment does not occur instantaneously upon talin unfolding, as assumed for simplicity in our previous models on elastic substrates. Instead, here we explicitly consider the timescale of this process, captured by the model via a stochastic, binding rate of vinculin to unfolded talin. In this scenario, clutch dynamics in the stiff condition become faster than vinculin binding, decreasing integrin recruitment (Fig. 2g). In summary, the model closely recapitulates the experimental effects of stiffness and viscosity.
Regarding the effects of Piezo1, different works have shown that it is involved in integrin activation and integrin adhesion maturation14. We thus modelled it by increasing integrin unbinding rates (\(k\)off) by 15% in siPiezo1 MSCs. With this, we reproduced the experimental effects of siPiezo1, which reduced integrin recruitment induced by both increased stiffness (soft V− versus stiff V−) and increased viscosity (soft V− versus soft V+). In the simulations, the viscous effect for low stiffness substrates is not entirely abolished. Whereas a more pronounced reduction of \(k\)off would allow for closer matching the experimental data on integrin densities, the results for the retrograde flow (Fig. 3) would not be consistent with experimental results. Overall, the modified clutch model (Fig. 2g) closely matches our experimental observations (Fig. 2d), which (i) describe a stiffness-dependent response to clutch engagement in viscoelastic matrices, (ii) highlight how Piezo1 regulates clutch activation and (iii) propose Piezo1 a key mediator of cell response to soft matrix viscoelasticity.
Fig. 3: Actin retrograde flow in response to matrix viscoelasticity is stiffness and Piezo1-dependent.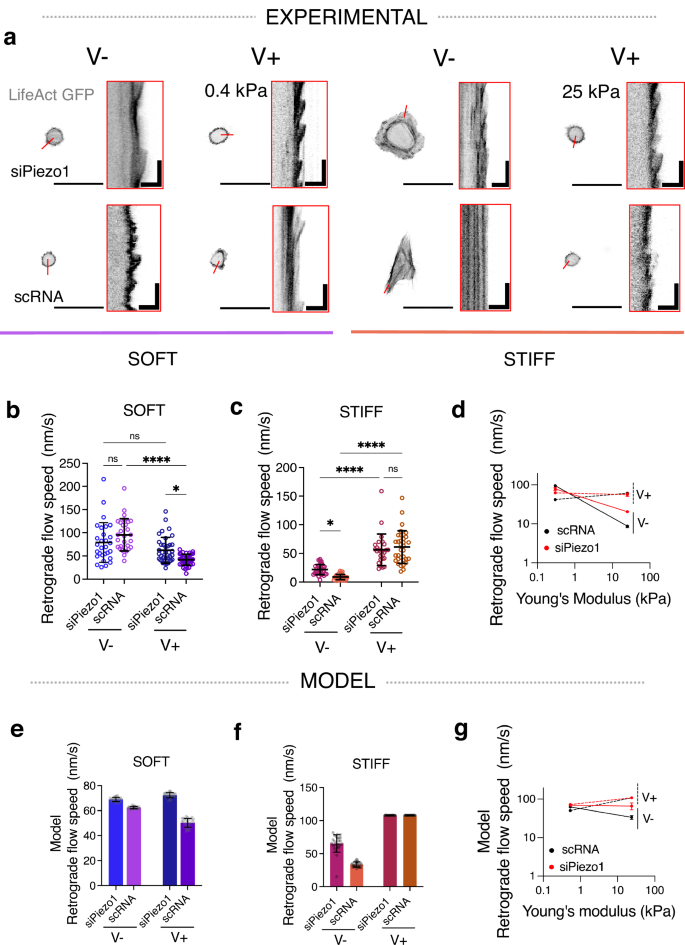
a Representative images of LifeAct GFP transfected siPiezo1 and scRNA MSCs cultured on the soft (left) and stiff (right) hydrogel groups. Scale bar = 50 µm. Red insets are kymographs showing the movement of actin features, scale bar = 5 µm (horizontal) and 60 s (vertical). b, c Quantified retrograde flow speed (nm/s) in siPiezo1 and scRNA MSCs cultured on the soft (left) and stiff (right) hydrogel groups. In (b) from left to right n = 29, 29, 38 and 39 individual kymograph measurements from 13, 8, 16 and 9 cells; in (c) from left to right n = 35, 39, 26 and 34 individual kymograph measurements from 12, 16, 13 and 17 cells. Data from N = 2 independent experiments. Data shown as individual values, mean ± SD. d Summary of mean experimental retrograde actin flow speed (nm/s) ± SEM plotted as a function of stiffness for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions. e, f Model predictions of retrograde flow speed (nm/s) of cells on soft (left) and stiff (right) substrates. Data shown as individual values, mean ± SD (N = 26 simulations). g Summary of model predictions of mean retrograde actin flow speed (nm/s) ± SEM plotted as a function of stiffness for scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line) conditions. Statistical analyses were performed using a two-way ANOVA test. P values indicating significance, ns > 0.05, *≤0.05, ***≤0.001, ****≤0.0001. Specific p values and descriptive statistics are provided in the Source Data.
FA maturation and size are inversely related to the actin retrograde flow rate in cells33, because when the molecular clutch is engaged via actin-talin-integrin-FN links, actin is bound in its fibrillar form and the rate of actin polymerisation decreases. We therefore transfected siPiezo1 and scRNA MSCs with Life-Act GFP, which stains filamentous actin (F-actin) structures (Fig. 3a), and measured the rate of actin retrograde flow using live confocal microscopy, in response to varying viscoelasticity on soft and stiff substrates. In accordance with FA data (Fig. 2b–d), actin retrograde flow was slowed in scRNA MSCs in response to faster substrate stress relaxation in soft hydrogels (Fig. 3b), whereas on stiff hydrogels, actin retrograde flow increased ~7 fold from a slow-relaxing (stiff V−) to a fast-relaxing (stiff V+) matrix in scRNA MSCs and ~3 fold in siPiezo1 MSCs (Fig. 3c). Notably, in siPiezo1 MSCs, the knocking down of Piezo1 abrogated any change in retrograde flow speed between V− and V+ conditions in the soft group (Fig. 3b). These data (Fig. 3b–d) reiterate the role of Piezo1 in finely sensing the time-dependent mechanics of soft matrices and in promoting clutch engagement in response to enhanced viscoelasticity on soft matrices.
Using the same modified clutch model as before (Fig. 2e), we assessed how stress relaxation and Piezo1 activity influence the retrograde actin flow speed of cells on soft (Fig. 3e) and stiff (Fig. 3f) hydrogels. Again, the modified clutch model (Fig. 3g) closely recapitulated our experimental observations (Fig. 3d), whereby on soft matrices, scRNA cells decrease actin flow rate in response to enhanced stress relaxation whereas on stiff substrates, scRNA MSC actin retrograde flow speed is increased. Moreover, on soft matrices, siPiezo1 cells did not reduce actin retrograde flow speed in response to enhanced energy dissipation, which proposes Piezo1 as a key regulator of stress relaxation-generated clutch activation at this stiffness.
Finally, to investigate how the observed adhesion phenotype and actin polymerisation might translate into cell-exerted forces transmitted to the underlying ECM, we quantified the average cell traction forces exerted by scRNA and siPiezo1 MSCs on the different (soft, stiff, V−, V+) substrate types through traction force microscopy (TFM). Substrates were prepared using 200 nm fluorescent beads, the displacement of which was calculated before and after cell-applied deformations and then converted into forces. We note that using an elastic algorithm likely overestimates forces on viscoelastic V+ substrates, as it does not account for the dissipation of exerted tractions (Supplementary Data Fig. 4a–d). Nevertheless, standard TFM has proved to be a useful tool for investigating relative changes in cell-exerted forces on viscoelastic PAAm hydrogels27. Accordingly, we found that on soft substrates, scRNA MSCs exhibited increased traction force generation in conditions of increased substrate stress relaxation (V+), whereas this increased traction force generation was abrogated in siPiezo1 MSCs (Supplementary Data Fig. 4b). On stiff substrates, both siPiezo1 and scRNA MSCs generated significantly lower average traction forces as substrate stress relaxation increased (Supplementary Data Fig. 4c). This response was further reduced in siPiezo1 MSCs, highlighting the role of Piezo1 in traction force generation mechanisms within the cell, consistent with previous reports16. Perturbation studies also highlighted this relationship; where inhibiting cell contractility with blebbistatin induced similar cell-substrate interaction phenotypes as those produced by siPiezo1 MSCs (Supplementary Data Fig. 5a, b).
Overall, our results underscore the role of Piezo1 in mediating viscoelasticity-sensing in soft but not stiff ECMs, in which Piezo1 knock down lessened the cell response to increased substrate stress relaxation but did not fully inhibit it. Indeed, our experimental data agrees with the molecular clutch dynamics in response to matrix viscoelasticity that were first proposed by Chaudhuri and colleagues9, in which clutch engagement is enhanced by substrate stress relaxation on soft environments but is inhibited above a stiffness threshold (E ~ 1 kPa)9,22. Considering these results, we explored how Piezo1 mediates clutch dynamics in cells interacting with a viscoelastic substrate (Fig. 2e). We hypothesised that Piezo1-integrin concerted action is pivotal for clutch engagement in response to i) faster stress relaxation at low stiffness (soft V+) as well as ii) high stiffness conditions with slow stress relaxation (stiff V−). We consolidated this hypothesis by modifying the clutch model to introduce the effect of Piezo1 within the viscoelastic molecular clutch framework. By representing the coordinated action of Piezo1 and integrins via the modulation of integrin-ECM unbinding rates (koff), we were able to reproduce experimental results. Model data thus supports our hypothesis that places Piezo1 as a key regulator of clutch engagement in response to viscoelastic cues, particularly in soft regimes.
Matrix viscoelasticity and Piezo1 regulate downstream mechanotransduction and mitochondrial morphology
We next investigated whether the observed changes in clutch engagement as a function of Piezo1 expression and substrate viscoelasticity activated downstream mechanotransduction events. Increased cytoskeletal tension has been associated with nuclear compression and chromatin compaction34,35. Because of this, we assessed and quantified nuclear projected spreading area (Fig. 4a, b, f). We observed that nuclear spreading was higher in scRNA MSCs cultured on soft, faster stress relaxation (soft V+) hydrogels compared to nuclear spreading in scRNA MSCs cultured on elastic (soft V−) hydrogels (Fig. 4a). Contrarily, nuclear spreading was reduced in scRNA MSCs cultured on stiff V+ hydrogels compared to stiff V− ones (Fig. 4b). Notably, in siPiezo1 MSCs, this increased nuclear spreading was abrogated on both soft V+ and stiff V− hydrogels (Fig. 4f), indicating that viscoelasticity and Piezo1-mediated cytoskeletal tension directly act on the nucleus.
Fig. 4: Matrix viscoelasticity and Piezo1 expression regulate downstream mechanotransduction and mitochondrial morphology.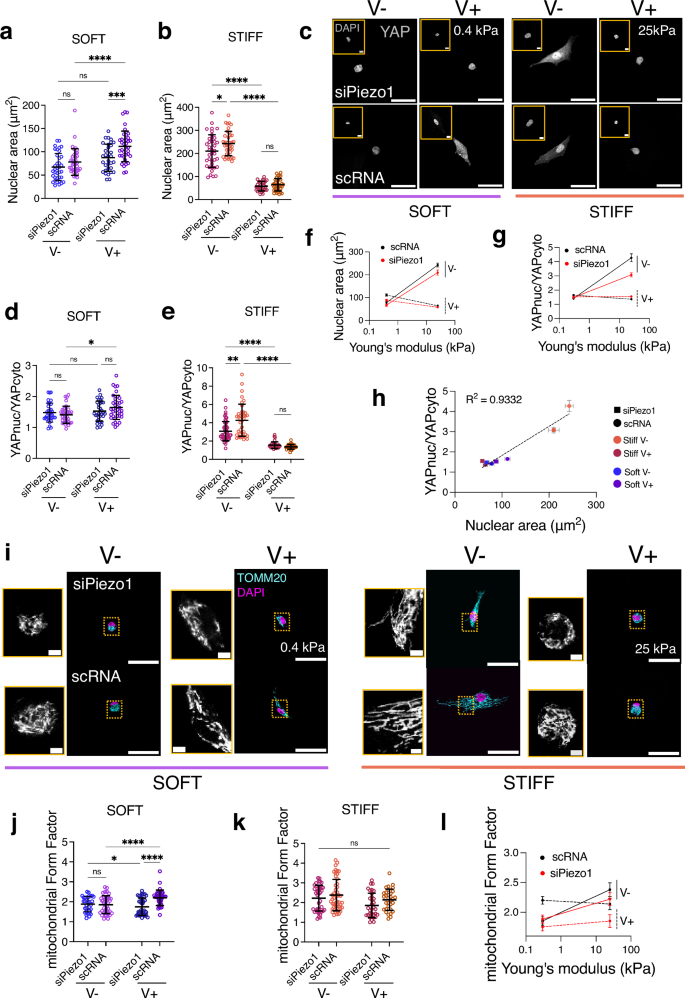
a, b Quantified nuclear spreading area on the soft (left) and stiff (right) hydrogel groups. In (a) from left to right, n = 36, 37, 32 and 38 cells; in (b) from left to right, n = 39, 38, 31 and 35 cells. Data from N = 3 independent experiments. Data shown as individual values, mean ± SD. c Representative images of nuclei (insets) and YAP in siPiezo1 (top) and scRNA Y201 MSCs (bottom) cells on soft (0.4 kPa, left) and stiff (25 kPa, right) hydrogel groups of varying stress relaxation. Scale bar = 50 µm, DAPI inset scale bar = 5 µm. d, e Quantified nuclear over cytoplasmic YAP (nucYAP/cytoYAP) ratio on the soft (left) and stiff (right) hydrogel groups. In (d) from left to right, n = 34, 34, 32 and 38 cells; in (e) from left to right n = 55, 40, 34 and 31 cells from N = 3 independent experiments. Data shown as individual values, mean ± SD. f Summary of mean nuclear spreading area ± SEM plotted as a function of stiffness for all conditions. g Summary of mean nucYAP/cytoYAP ratio ±SEM plotted as a function of stiffness for all conditions. In (f, g) conditions are as follow: scRNA (black), siPiezo1 (red), V− (continuous line) and V+ (dashed line). h Correlation between nucYAP/cytoYAP and nuclear spreading area for all conditions. scRNA conditions have rounded symbols whilst siPiezo1 are squared. Symbol colour indicates stiffness and energy dissipation conditions. Data shown as mean ± SEM. R2 obtained from simple linear regression analysis fitting mean values i Representative images of siPiezo1 (top) and scRNA (bottom) MSCs immunostained for TOMM20 (cyan) and DAPI (magenta) cultured on soft (0.4 kPa, left) and stiff (25 kPa, right) hydrogels for 48 h. Scale bar 50 µm; TOMM20 inset scale bar 2 µm j, k Quantified mean mitochondrial form factor of MSCs cultured on the soft (left) and stiff (right) hydrogel groups. In (j) from left to right, n = 32, 40, 40 and 36 cells; in (k) from left to right, n = 37, 48, 33 and 32 cells. Data from N = 3 independent experiments. l Summary of mean mitochondrial form factor ± SEM plotted as a function of stiffness for all conditions. a, b, d, e, j, k Statistical analyses were performed using a two-way ANOVA test. P values indicating significance, ns > 0.05, *≤0.05, **≤0.01, ***≤0.001, ****≤0.0001. Specific p values and descriptive statistics are provided in the Source Data.
It has recently been proposed36,37 that the force applied to the nucleus could dictate the nuclear translocation of important transcription factors, such as Yes-associated protein (YAP), independently of other specific signalling pathways. Nuclear flattening (i.e. increased projected spreading area) increases nucleocytoplasmic transport, leading to the nuclear translocation of factors such as YAP through differential effects on active versus passive transport. Previous work has reported that Piezo1 activity is linked to enhanced YAP nuclear translocation18 and on the importance of Piezo1 in sensitively sensing tensional changes to regulate nuclear size in response to exogenously applied shear stress38. Thus, we sought to understand whether the observed morphological changes in the nucleus reflected transcription factor translocation by assessing the localisation of YAP (Fig. 4c). In both siPiezo1 and scRNA MSCs, YAP translocated into the nucleus (YAPnuc/YAPcyto > 2) in response to increased substrate stiffness (Fig. 3d, e, g). However, on soft substrates, YAP was mostly cytoplasmic in siPiezo1 and scRNA MSCs, and did not translocate in response to increased stress relaxation (V+). YAP nuclear translocation in response to molecular clutch activation mechanisms has been shown to occur past an elasticity threshold of E ~ 5 kPa6. Therefore, soft viscoelastic (soft V+) substrates did not promote sufficient adhesion maturity and reinforcement, or stress fibre formation to facilitate YAP nuclear localisation (nucYAP/cyto YAP 39. When siPiezo1 MSCs were cultured on stiff substrates with faster substrate relaxation (stiff V+), both nuclear spreading area and YAP translocation were reduced. Similarly, on stiff V− substrates, siPiezo1 MSCs showed significantly lower levels of nuclear YAP (Fig. 4e). Likewise, when siPiezo1 MSCs were cultured on FN-coated glass, we observed smaller nuclei (Supplementary Data Fig. 6b) as well as slightly reduced YAP nuclear translocation (Supplementary Data Fig. 6a, c), when compared to scRNA MSCs. These results concur that regardless of the substrate employed, reducing Piezo1 expression generally decreases YAP nuclear translocation.
To further corroborate this relationship, plotting the mean nucYAP/cytoYAP against the mean nuclear spreading area across all experimental conditions revealed a strong positive correlation between the two variables (Fig. 4h, R2 = 0.9332). These results highlight that YAP is sensitive to both the effects of viscoelasticity and Piezo1-mediated cytoskeletal tension, which both act on the nucleus starting from the molecular clutch.
We then investigated how the cell-substrate interaction affected the cell’s metabolic capacity, as recent evidence has linked stiffness-dependent integrin signalling to the modulation of mitochondrial activity40. We performed preliminarily oxygen consumption rate (OCR) experiments on stiff and soft Matrigel substrates (Supplementary Data Fig. 7a). We used Matrigel substrates in these experiments due to the technical implications of using the Seahorse assay to measure OCR, which made implementing viscoelastic PAAm substrates complex owing to having to polymerise the different hydrogels on a coated 96 well plate format. By contrast, soft (E ~ 0.2 kPa) and stiff (E ~ 1 GPa) Matrigel systems have previously been applied into the seahorse assay experimental set-up41. OCR measurements indicated that Y201 MSC mitochondrial respiration rates were sensitive both to substrate stiffness and Piezo1 expression (Supplementary Data Fig. 7b–d). Conversely, non-mitochondrial respiration was only significantly decreased when Piezo1 was knocked down on stiff Matrigel substrates (Supplementary Data Fig. 7e). We found that a soft matrix decreased cellular respiration capacity independently of Piezo1 expression (Supplementary Data Fig. 7a). However, on stiff substrates, mitochondrial-dependent respiration was increased, but this increase was abrogated if Piezo1 was knocked down (Supplementary Data Fig. 7e). Our data thus indicate that mitochondria are targeted by the Piezo1-modulated mechanosensing of matrix mechanics, regulating cellular respiration. Therefore, in order to assess mitochondrial respiration in response to varying viscoelasticity in our experimental set up, we labelled the outer mitochondrial membrane protein TOMM20 via immunostaining (Fig. 4i) and assessed mitochondrial elongation by quantifying the mean mitochondrial Form Factor in all experimental conditions (Fig. 4j–l). Mitochondrial elongation provides information on the fusion vs fission events in the organelle, alluding to the proliferative and respiratory state of the cell. In the past, mitochondrial fission and fusion has been shown to be significantly altered in response to changes in substrate stiffness42. Indeed, mitochondria appeared shorter on soft elastic (soft V-) compared to stiff elastic (stiff V-) substrates, as previously reported in ref. 40. Increased stress relaxation on soft hydrogels promoted mitochondrial elongation and abrogated matrix-induced mitochondrial fission (Fig. 4j). Whereas on stiff substrates (Fig. 4k), mitochondria were most elongated on scRNA cells cultured on slow stress relaxing substrates (stiff V−). In this case, both stress relaxation and Piezo1 knock down conditions decreased mitochondrial form factor. Besides mitochondrial elongation, we also quantified the total mitochondrial area in the cell, to estimate respiration rates42. Indeed, from total mitochondrial area data (Supplementary Data Fig. 8), it is possible to hypothesise that overall cell respiration is highly linked to mitochondrial mass inside the cell. This means that increased substrate relaxation (V+) enhances cellular respiration and metabolism in soft ECMs (Supplementary Data Fig. 8a), whereas on stiff matrices (Supplementary Data Fig. 8b), increased stress relaxation decreases overall cellular respiration and slows the cell’s metabolic capabilities. Still, it does not significantly reduce mitochondrial form factor (Fig. 4k), suggesting that cells are not under oxidative stress. In terms of Piezo1’s role in mitochondrial dynamics, it appears that its knock down generally promotes mitochondrial fission (Fig. 4l). This is most likely a consequence of the Piezo1’s role in mediating the cell’s interaction with its environment. Thus, mitochondrial fission induced by Piezo1 knock down is a read-out of the effects of Piezo1 on overall cell morphology.
Matrix viscoelasticity and Piezo1’s influence on transcriptomic phenotype
Finally, to monitor matrix viscoelasticity and Piezo1 activity-dependent transcriptional changes and to obtain reference transcriptomic phenotypes, we performed RNAseq on all experimental conditions at the 48 h timepoint of the previously shown mechanotransduction and metabolic data. This timepoint was chosen for two reasons: (i) to compare RNAseq data with previously shown data; and (ii) because in previous studies of MSC responses to varying stiffness, stress relaxation and ligand density in 3D matrices, RNAseq was performed at 40 h to ensure mature adhesions had formed with minimal cell proliferation43,44. Prior to assessing differential gene expression in response to viscoelastic matrices, we ensured that Piezo1 knock down was efficient in cells used for sequencing, to control for experimental upscaling. For this, scRNA and siPiezo1 cells were cultured on FN coated glass and RNAseq was performed at the 48 h timepoint. Gene counts for Piezo1 were reduced in siPiezo1 cells, with a significant fold change after performing differential gene expression analysis (Supplementary Data Fig. 9a, b). Further, we saw a clear transcriptome change from scRNA to siPiezo1 cells, where 66 genes were differentially expressed (p 9c). We also performed gene ontology (GO) enrichment analysis on the DE genes between de siPiezo1 and scRNA comparison. GO enrichment places top DE genes in functional modules of relevant sub-ontologies45,46,47. GO enrichment by over-representation analysis (ORA) highlighted cell adhesion as the top differentially expressed biological process (Supplementary Data Fig. 9d), supporting literature evidence of a Piezo1-integrin concerted action14,15,16,17. These findings also underpin the hypothesis of our work, which places Piezo1 as a key mediator of adhesion complexes, and thus of molecular clutch dynamics at the cell-ECM interface.
We then performed differential expression analysis on the resulting RNAseq data from both siPiezo1 and scRNA MSCs cultured on our hydrogel substrates and generated a curated heatmap with z-score up- and down-regulated genes plotted for each hydrogel stiffness range (Fig. 5a and Fig. 6a) as well as a general differential expression heatmap of genes with p 10). Curated family heatmaps display z-score rlog-normalised expression for genes from five functionally defined relevant gene families: integrins, actin, YAP, mitochondria and contractility, which were used to create a visual representation of inner stiffness-group transcriptome variation. From these data it is possible to discern the strong interplay between Piezo1, integrins, actin cytoskeleton and myosin-based contractility mechanisms in the cell at this stiffness range (25 kPa); as Piezo1 knock down generally decreases gene expression within these functional families (Fig. 5a). Also, in accordance with our data, integrin, actin and contractility subfamilies are generally overexpressed in V– conditions, and levels decrease in response to enhanced energy dissipation (V+). Additionally, we assessed mitochondrial and YAP functional groups. The YAP subfamily is overexpressed in scRNA V−, with both Piezo1 knock down and energy dissipation having a downregulatory effect, as shown in Fig. 4e. Further, we observed that Piezo1 knock down seems to produce a decrease in mitochondrial genes, as demonstrated experimentally in Fig. 4k. Finally, a general downregulation of functional gene families is observed in response to enhanced energy dissipation at this stiffness.
Fig. 5: RNA-seq analysis of siPiezo1 and scRNA Y201 MSCs cultured on stiff elastic (V−) and viscoelastic (V+) matrices.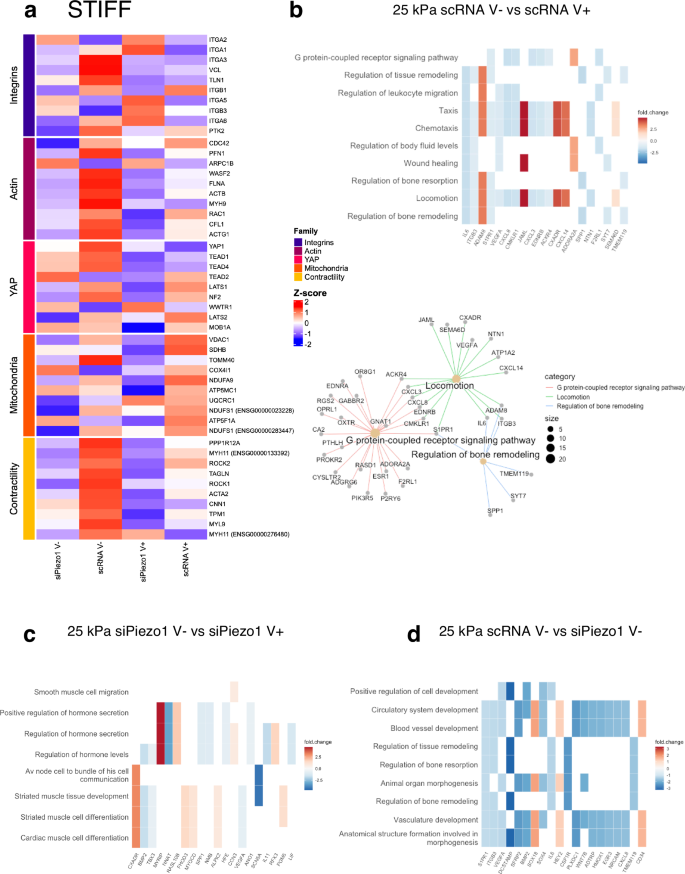
a Curated z-score (rlog-normalised expression) heatmap of stiff group genes across experimental conditions per defined gene families (Integrins, Actin cytoskeleton, YAP signalling, Mitochondria and Contractility). b (Top) Heatmap of enriched results from Over Representation Analysis (ORA), which overlap with the most DE genes in the scRNA MSC V− vs scRNA MSC V+ comparison. (Bottom) A network of the three top enriched DE gene groups connected by their overlapping genes. c Heatmap of enriched results from ORA that overlap with the most DE genes in the siPiezo1 V− vs siPiezo1 V+ comparison. d Heatmap of enriched results from ORA that overlap with the most DE genes in the scRNA V− vs siPiezo1 V− comparison.
Fig. 6: RNA-seq analysis of siPiezo1 and scRNA Y201 MSCs cultured soft elastic (V−) and viscoelastic (V+) matrices.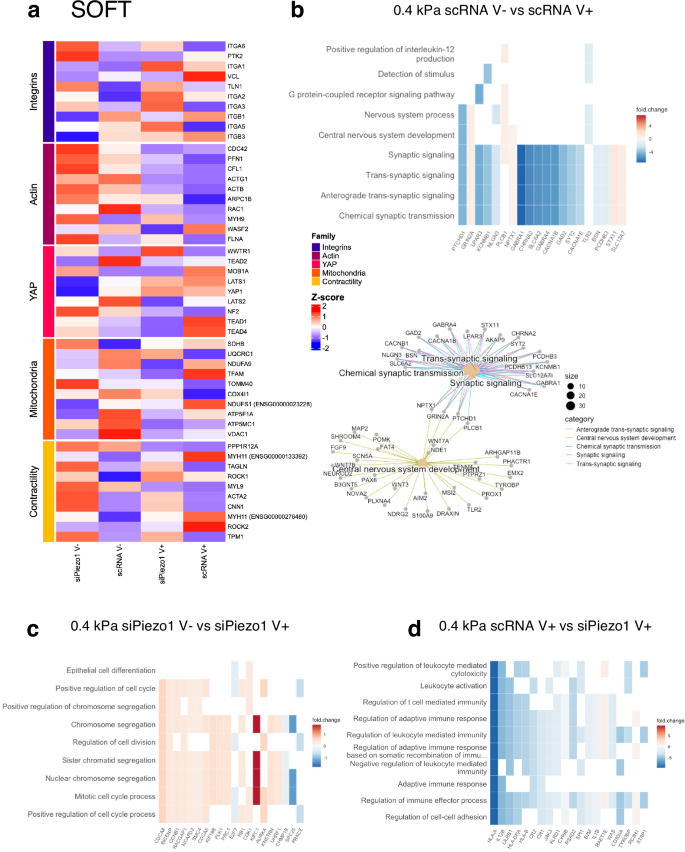
a Curated z-score (rlog-normalised expression) heatmap of soft group genes across experimental conditions per defined gene families (Integrins, Actin cytoskeleton, YAP signalling, Mitochondria and Contractility). b (Top) Heatmap of enriched results from Over Representation Analysis (ORA), which overlap with the most DE genes in the scRNA MSC V− vs scRNA MSC V+ comparison. (Bottom) A network of the top enriched DE gene groups connected by their overlapping genes. c Heatmap of enriched results from Gene Set Enrichment Analysis (GSEA) that overlap with the most DE genes in the siPiezo1 V− vs siPiezo1 V+ comparison. d Heatmap of enriched results from ORA that overlap with the most DE genes in the scRNA V− vs siPiezo1 V− comparison.
We expanded our analysis to perform GO enrichment between experimental conditions. First, we assessed the enrichment of the DE genes from scRNA cells seeded on stiff elastic (V−) versus viscoelastic (V+) matrices to assess the processes that drive Y201 MSCs’ response to viscoelasticity at this stiffness (Fig. 5b). The top sub ontologies to emerge from this GO analysis were cell motility, bone remodelling and G-protein-coupled receptor (GPCR) signalling. These are processes that are associated in the literature with cell interactions in stiff 2D viscoelastic substrates. For example, our group has demonstrated that cell motility is reduced in breast epithelial cells in response to enhanced substrate stress relaxation at E > 1 kPa48. Similarly, at stiffness E > 1 kPa, we have shown that viscoelasticity reduces cell spreading area, molecular clutch engagement and YAP nuclear translocation, which are all processes involved in bone remodelling. In previous work by our group, we have demonstrated that substrate stress relaxation in hydrogels of similar stiffness (E ~ 13 kPa) promoted chondrogenesis in hMSCs25. Finally, the GPCR pathways ontology includes genes that belong to the Ras-subfamily of GTPases (RASD1) or Rho signalling (GNAT1), which mediate processes that have been previously highlighted in studies describing cell response to 2D viscoelastic substrates24.
We performed the same comparison on siPiezo1 MSCs to investigate Piezo1-independent transcriptional mechanisms of matrix viscoelasticity for the stiff substrate. In this GO analysis, processes such as hormone secretion and regulation, muscle tissue development and differentiation and smooth muscle cell migration were highlighted (Fig. 5c). From these results, we hypothesise that Piezo1 expression is crucial for mediating viscoelasticity-induced changes in bone remodelling and locomotion processes. However, when Piezo1 is silenced in Y201 MSCs, enhanced substrate stress relaxation affects myosin-related ontologies (i.e. muscle tissue development and differentiation). These data are supported by our perturbation studies (Supplementary Data Fig. 5), where inhibiting Myosin-II mediated cell contractility with blebbistatin inhibited cell mechanoactivation to similar levels to those of Piezo1 knock down. Results thus suggest that myosin signalling mechanisms still respond to enhanced viscoelasticity, but that Piezo1 is required to further these adaptions into canonical mechanotransduction pathways, as highlighted in Fig. 5b. This concomitant action between myosin signalling and Piezo1 has also been highlighted previously16, and the interplay between Piezo1 and cell-generated forces is key to allow for the spatial segregation of mechanotransduction events such as locomotion, bone remodelling and GPCR signalling.
Finally, we compared transcriptomic data from scRNA vs siPiezo1 MSCs cultured on stiff elastic (25 kPa, V−) matrices (Fig. 5d). The top sub ontologies to emerge from this GO analysis of the top DE genes included cardiovascular system remodelling, bone remodelling and morphogenesis. Piezo1 knockdown downregulated genes such as BMP2, VEGFA, ITGB3, which are involved in cell proliferation, differentiation and integrin-related signalling. In fact, these processes have been directly associated with Piezo1’s activity and expression in MSCs49, and support our hypothesis that Piezo1 knock down reduces overall molecular clutch engagement (Fig. 2c and Fig. 3d) on stiff elastic matrices and glass substrates (Supplementary Data Fig. 2c, d and Supplementary Data Fig. 9d), as well as reducing YAP nuclear translocation (Fig. 4g and Supplementary Data Fig. 6c), which has been shown to regulate bone differentiation in MSCs50.
We conducted the same analysis for scRNA vs siPiezo1 MSCs cultured on soft substrates, and identified 177 DE genes (Supplementary Data Fig. 11), which suggests that when MSCs are cultured on soft substrates both Piezo1 expression and substrate energy dissipation produce a less pronounced effect on the transcriptome than when MSCs are cultured on stiff substrates. A curated heatmap of rlog normalised gene expression across relevant gene families was plotted (Fig. 6a). Here, in the scRNA V+ conditions, genes such as vinculin (VCL), integrin β1 and β3 (ITGB1 and ITGB3), Myosin11 (MYH11) and ROCK2 show overexpression; highlighting their role in mediating the mechanoactivation we observed experimentally in response to enhanced energy dissipation at this stiffness. Accordingly, these all became downregulated in siPiezo1 cells. Additionally, increased energy dissipation on scRNA MSCs upregulated YAP1 and TEAD associated genes, commensurate with data shown in Fig. 4, d, where we show that although YAP does not become nuclear at this stiffness in response to increased energy dissipation, there is an increase in mechanotransductive mechanisms. Finally, in our mitochondrial gene family, two mitochondrial biogenesis genes (TFAM and NDUFS1) were overexpressed in scRNA V+ conditions, indicating that enhanced energy dissipation at this stiffness potentially increases the metabolic capacity of cells without inducing mitochondrial membrane damage; this is in line with data we showed in Fig. 4j. and Supplementary Fig. 8.
We then assessed and compared enriched DE genes in scRNA MSCs cultured on soft elastic (soft V−) versus on viscoelastic (soft V+) matrices (Fig. 6b). The top sub ontologies to emerge from this comparative GO analysis were the GPCR pathway, synaptic signalling and central nervous system development. Interestingly, in the central nervous system development subontology, we found the Wnt family genes, WNT7A and WNT3, which are important regulators of YAP and respond to matrix stiffness51. Although at this stiffness range (~0.4 kPa), viscoelasticity did not promote YAP nuclear translocation in cells (Fig. 4f), it is possible that components of the non-canonical Wnt signalling pathway52, such as WNT3 and GPCRs (Gα subunits), still become activated to support cell proliferation in response to enhanced substrate stress relaxation. These sub-ontologies have been highlighted in previous studies that investigated transcriptional changes in MSCs encapsulated in dynamic viscoplastic matrices compared to fully crosslinked elastic matrices53. Additionally, we found that the synaptic signalling sub-ontology was underscored in the enrichment analysis. Indeed, genes in this subontology include phospholipase C beta 1 (PLCB1), which is associated with cytoskeletal rearrangement processes in gastric tumour tissue samples54 and with other genes that encode Ca2+ responsive channels (such as CACNA1E, CACNA1B and KCNMB1). These results could explain our data on scRNA MSC enhanced cell spreading area (cytoskeletal rearrangement) and clutch engagement (Fig. 1g; Fig. 2b and Fig. 3b) in response to increased viscoelasticity on soft matrices.
As before, we compared transcriptomic data from siPiezo1 MSCs cultured on soft elastic (soft V−) vs on soft viscoelastic (soft V+) matrices to highlight the Piezo1-dependent cell response to viscoelasticity at this stiffness. Notably, our DE gene enrichment analysis showed that most of the previously identified GO sub-ontologies were replaced by cell division processes, which were mostly up regulated in viscoelastic hydrogels in a Piezo1-independent manner. In our data, we do not see any phenotypic difference between siPiezo1 MSCs cultured on soft elastic (V–) vs viscoelastic (V+) hydrogels. Nonetheless, it might be that viscoelasticity in soft matrices promotes cell proliferation, as previously reported in a cancer cell line9 and this may happen independently of Piezo1.
We compared transcriptomic data from scRNA and siPiezo1 MSCs cultured on soft viscoelastic (V+) hydrogels. Particularly, Piezo1 knock down promoted the down regulation of several ontologies relating to immune response regulation and cell-cell adhesion. MSCs have important immunomodulatory properties, which are responsive to matrix mechanics55,56. Similarly, Piezo1 mechanosensing has recently been linked to adherens cell-cell junction formation in endothelial cells57,58, as well as to immune cell migration59. However, the link between Piezo1 knock down and MSC downregulation of immunomodulatory genes remains unclear, and something that should be addressed in future work. Interestingly, in previous analyses of transcriptomic changes in MSCs encapsulated in 3D hydrogels of increasing stiffness (in which E increased from 3 kPa to 18 kPa), several immunomodulatory markers were differentially expressed43. This was found to be linked to stiffness-induced activation of the immune and inflammatory transcription factor NFkB-p65. Previous work from our group55,60 has addressed that MSCs reach an immunomodulatory phenotype in intermediate adhesion states, where adhesion – and thus- molecular clutch mechanisms are active but not so overly engaged to promote differentiation; from these results we propose that Piezo1 is a mediator of stiffness-induced immunomodulation in MSCs on softer substrates.
Overall, our transcriptomic analyses indicate that both viscoelasticity and Piezo1 activity regulate a diverse range of processes in MSCs, and that depending on substrate stiffness, different gene groups respond to changes in matrix viscoelasticity as well as to Piezo1 knock down.