[Illustration: beatpavel/Shutterstock]
A new study from UT Southwestern suggests there may be a biological reason so many people find themselves reaching for snacks at night. And it may start in a part of the brain that keeps time.
Researchers at UTSW point to a set of neurons in the brain’s “master circadian pacemaker”—the suprachiasmatic nucleus—that appear to influence hunger and metabolism.
“We identify for the first time a distinct set of neurons in the brain that controls feeding and metabolism during one specific time of day,” said Dr. Jeffrey Zigman, co-leader of the study and professor of Internal Medicine and Psychiatry at UT Southwestern.

Jeffrey Zigman, M.D., Ph.D. [Photo: UTSW]
In a news release, Zigman says researchers know “eating late at night is associated with greater weight gain than eating the same amount during the day. The effect is most apparent in night shift workers “who are more frequently overweight or obese despite caloric intake similar to day workers.”
Zigman co-led the study focused on neurons in the “brain’s timekeeper” that might control nighttime hunger with postdoctoral researcher and first author Omprakash Singh. Their study, published in Cell Reports, showed that flipping these neurons on or off in mice directly affected how much they ate—and how much they weighed—during their usual rest period.
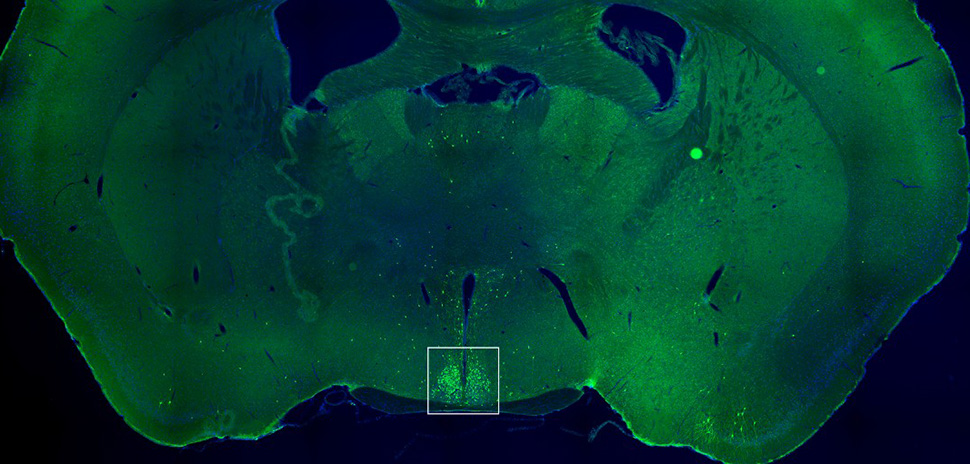
In this slice of a mouse brain, the white box highlights the suprachiasmatic nucleus—the brain’s internal clock. The green dots mark neurons that respond to the hunger hormone ghrelin. [Image: UTSW]
Using genetically altered mice, UTSW said the researchers were able to activate or silence SCN neurons that respond to ghrelin, a hormone known to stimulate appetite and slow metabolism. When the neurons were turned on during the animals’ rest period, the mice ate more than twice as much as usual. When the neurons were turned off, snacking dropped, and weight loss followed.
After 15 days of daily neuron suppression during rest hours, the mice lost an average of 4.3% of their body weight, according to UTSW. Mice with unaltered neurons gained about 2.5% over the same period—a swing of nearly 7% in body weight driven by timing alone.
The discovery could eventually help inform new strategies for weight management, particularly for night shift workers and others prone to late-night eating. Researchers say it might one day lead to new therapies that modulate this time-specific neural circuit instead of relying solely on medications that act on the whole body.
If the results apply to humans, Zigman said, targeting “this population of neurons” could offer weight-loss benefits similar to some modern drugs.
Don’t miss what’s next. Subscribe to Dallas Innovates.
Track Dallas-Fort Worth’s business and innovation landscape with our curated news in your inbox Tuesday-Thursday.
R E A D N E X T
-
UT Dallas researcher Dr. Walter Voit transformed Minecraft’s 170-million-player universe into an advanced virtual training ground—for students and for AI agents tested by DARPA. His team’s Polycraft World uses gameplay to turn classroom theory into real-world expertise, covering topics from synthetic organic chemistry to nuclear plants to semiconductor facilities. Their new startup company, Pedegree Studios, has licensed the core technologies from the university to create a scalable digital pipeline for education and workforce development.
-

The Building Brains Coalition builds upon the growing recognition that brain health is integral to human potential and societal progress, HKS said. Founding partners include HKS, The Center for BrainHealth, Center for Advanced Design Research and Evaluation (CADRE), the Brain Capital Alliance, and Rice University’s Baker Institute Neuro-Policy Program.
-

Dr. Kirk Calhoun was named interim president following the resignation in January of UNTHSC President Sylvia Trent-Adams.
-
A UT Southwestern study of blind fruit flies and mice reveals a brain-wide stress response, shedding light on the biological link between sensory loss and dementia.
-

Dallas biotech GenrAb is developing an antibody therapy that aims to protect brain cells under stress—the kind of damage that leads to disability in diseases like MS and ALS. The therapy, found in the spinal fluid of real patients, is now showing promise in animal models and moving toward human trials.