Thudichum JLW. A treatise on the chemical constitution of the brain: Bailliere, Tindall, and Cox, London; 1884.
Chiricozzi E, Aureli M, Mauri L, Di Biase E, Lunghi G, Fazzari M, et al. Glycosphingolipids. Adv Exp Med Biol. 2021;1325:61–102.
Schnaar RL. The biology of gangliosides. Adv Carbohydr Chem Biochem. 2019;76:113–48.
Inokuchi JI, Inamori KI, Kabayama K, Nagafuku M, Uemura S, Go S, et al. Biology of GM3 ganglioside. Prog Mol Biol Transl Sci. 2018;156:151–95.
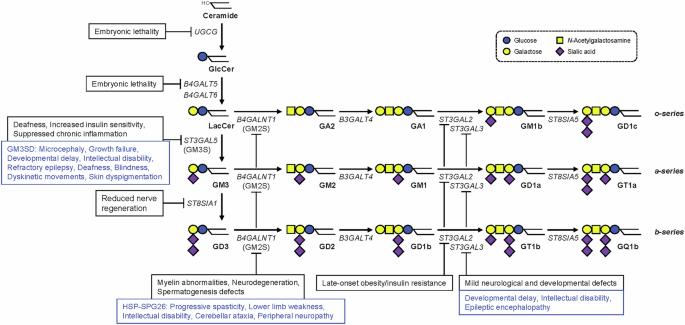
Ishii A, Ohta M, Watanabe Y, Matsuda K, Ishiyama K, Sakoe K, et al. Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. J Biol Chem. 1998;273:31652–5.
Nagata Y, Yamashiro S, Yodoi J, Lloyd KO, Shiku H, Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992;267:12082–9.
Harlalka GV, Lehman A, Chioza B, Baple EL, Maroofian R, Cross H, et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain. 2013;136:3618–24.
Boukhris A, Schule R, Loureiro JL, Lourenco CM, Mundwiller E, Gonzalez MA, et al. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am J Hum Genet. 2013;93:118–23.
Edvardson S, Baumann AM, Muhlenhoff M, Stephan O, Kuss AW, Shaag A, et al. West syndrome caused by ST3Gal-III deficiency. Epilepsia. 2013;54:e24–7.
Hu H, Eggers K, Chen W, Garshasbi M, Motazacker MM, Wrogemann K, et al. ST3GAL3 mutations impair the development of higher cognitive functions. Am J Hum Genet. 2011;89:407–14.
Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36:1225–9.
Bowser LE, Young M, Wenger OK, Ammous Z, Brigatti KW, Carson VJ, et al. Recessive GM3 synthase deficiency: Natural history, biochemistry, and therapeutic frontier. Mol Genet Metab. 2019;126:475–88.
Lee JS, Yoo Y, Lim BC, Kim KJ, Song J, Choi M, et al. GM3 synthase deficiency due to ST3GAL5 variants in two Korean female siblings: Masquerading as Rett syndrome-like phenotype. Am J Med Genet A. 2016;170:2200–5.
Boccuto L, Aoki K, Flanagan-Steet H, Chen CF, Fan X, Bartel F, et al. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum Mol Genet. 2014;23:418–33.
Wang H, Bright A, Xin B, Bockoven JR, Paller AS. Cutaneous dyspigmentation in patients with ganglioside GM3 synthase deficiency. Am J Med Genet A. 2013;161A:875–9.
Fragaki K, Ait-El-Mkadem S, Chaussenot A, Gire C, Mengual R, Bonesso L, et al. Refractory epilepsy and mitochondrial dysfunction due to GM3 synthase deficiency. Eur J Hum Genet. 2013;21:528–34.
Watanabe S, Lei M, Nakagawa E, Takeshita E, Inamori KI, Shishido F, et al. Neurological insights on two siblings with GM3 synthase deficiency due to novel compound heterozygous ST3GAL5 variants. Brain Dev. 2023;45:270–7.
Paulson JC, Rademacher C. Glycan terminator. Nat Struct Mol Biol. 2009;16:1121–2.
Gordon-Lipkin E, Cohen JS, Srivastava S, Soares BP, Levey E, Fatemi A. ST3GAL5-related disorders: a deficiency in ganglioside metabolism and a genetic cause of intellectual disability and choreoathetosis. J Child Neurol. 2018;33:825–31.
Indellicato R, Parini R, Domenighini R, Malagolini N, Iascone M, Gasperini S, et al. Total loss of GM3 synthase activity by a normally processed enzyme in a novel variant and in all ST3GAL5 variants reported to cause a distinct congenital disorder of glycosylation. Glycobiology. 2019;29:229–41.
Heide S, Jacquemont ML, Cheillan D, Renouil M, Tallot M, Schwartz CE, et al. GM3 synthase deficiency in non-Amish patients. Genet Med. 2022;24:492–8.
Mu D, Yang Y, Liu Y, Shen Y, Liu H, Wang J. Identification of a novel ST3GAL5 variant in a Chinese boy with GM3 synthase deficiency and literature review of variants in the ST3GAL5 gene. Orphanet J Rare Dis. 2024;19:423.
Rudy N, Aoki K, Ananth A, Holloway L, Skinner C, Hurst A, et al. Compound heterozygous variants within two conserved sialyltransferase motifs of ST3GAL5 cause GM3 synthase deficiency. JIMD Rep. 2023;64:138–45.
Abdulkareem AA, Shirah BH, Naseer MI. Whole exome sequencing reveals a novel homozygous variant in the ganglioside biosynthetic enzyme, ST3GAL5 gene in a Saudi family causing salt and pepper syndrome. Genes (Basel). 2023;14:354.
Manoochehri J, Dastgheib SA, Khamirani HJ, Mollaie M, Sharifi Z, Zoghi S, et al. A novel frameshift pathogenic variant in ST3GAL5 causing salt and pepper developmental regression syndrome (SPDRS): A case report. Hum Genome Var. 2021;8:33.
Meyyazhagan A, Orlacchio A. Hereditary spastic paraplegia: an update. Int J Mol Sci. 2022;23:1697.
Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18:1136–46.
Inamori KI, Nakamura K, Shishido F, Hsu JC, Nagafuku M, Nitta T, et al. Functional evaluation of novel variants of B4GALNT1 in a patient with hereditary spastic paraplegia and the general population. Front Neurosci. 2024;18:1437668.
Alecu JE, Ohmi Y, Bhuiyan RH, Inamori KI, Nitta T, Saffari A, et al. Functional validation of novel variants in B4GALNT1 associated with early-onset complex hereditary spastic paraplegia with impaired ganglioside synthesis. Am J Med Genet A. 2022;188:2590–8.
Dad R, Walker S, Scherer SW, Hassan MJ, Alghamdi MD, Minassian BA, et al. Febrile ataxia and myokymia broaden the SPG26 hereditary spastic paraplegia phenotype. Neurol Genet. 2017;3:e156.
Wakil SM, Monies DM, Ramzan K, Hagos S, Bastaki L, Meyer BF, et al. Novel B4GALNT1 mutations in a complicated form of hereditary spastic paraplegia. Clin Genet. 2014;86:500–1.
Wilkinson PA, Simpson MA, Bastaki L, Patel H, Reed JA, Kalidas K, et al. A new locus for autosomal recessive complicated hereditary spastic paraplegia (SPG26) maps to chromosome 12p11.1-12q14. J Med Genet. 2005;42:80–2.
Bhuiyan RH, Ohmi Y, Ohkawa Y, Zhang P, Takano M, Hashimoto N, et al. Loss of Enzyme Activity in Mutated B4GALNT1 Gene Products in Patients with Hereditary Spastic Paraplegia Results in Relatively Mild Neurological Disorders: Similarity with Phenotypes of B4galnt1 Knockout Mice. Neuroscience. 2019;397:94–106.
Giacomozzi S, Bonan L, La Morgia C, Carbonelli M, Santucci M, Isidori F, et al. Expanding the clinical spectrum of SPG26: a case report and review of B4GALNT1-associated hereditary spastic paraplegia. Mov Disord Clin Pract. 2025. https://doi.org/10.1002/mdc3.70062. Online ahead of print.
Yu W, He J, Liu X, Wu J, Cai X, Zhang Y, et al. Clinical features and genetic spectrum of Chinese patients with hereditary spastic paraplegia: A 14-year study. Front Genet. 2023;14:1085442.
Incecik F, Herguner OM, Bozdogan ST. Hereditary spastic paraplegia type 26 with a novel mutation in B4GALNT1 gene and literature review of the clinical features. J Paediatr Neurosci. 2023;18:354–6.
Wang C, Zhang YJ, Xu CH, Li D, Liu ZJ, Wu Y. The investigation of genetic and clinical features in patients with hereditary spastic paraplegia in central-Southern China. Mol Genet Genom Med. 2021;9:e1627.
Koh S, Lee SE, Jung WS, Choi JW, Lee JS, Hong JM, et al. Predictors of early neurological deterioration in stroke due to vertebrobasilar occlusion. Front Neurol. 2021;12:696042.
Rose L, Hall K, Tang S, Hasadsri L, Kimonis V. Homozygous B4GALNT1 mutation and biochemical glutaric acidemia type II: a case report. Clin Neurol Neurosurg. 2020;189:105553.
Indellicato R, Domenighini R, Malagolini N, Cereda A, Mamoli D, Pezzani L, et al. A novel nonsense and inactivating variant of ST3GAL3 in two infant siblings suffering severe epilepsy and expressing circulating CA19.9. Glycobiology. 2020;30:95–104.
Monies DA, Ibrahim J, Elbardisy N, Abouelhoda H, Meyer M, Alkuraya BF. F. S. Identification of a novel lethal form of autosomal recessive ichthyosis caused by UDP-glucose ceramide glucosyltransferase deficiency. Clin Genet. 2018;93:1252–3.
Jennemann R, Sandhoff R, Langbein L, Kaden S, Rothermel U, Gallala H, et al. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J Biol Chem. 2007;282:3083–94.
Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, et al. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96:9142–7.
Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, et al. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96:7532–7.
Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93:10662–7.
Inamori KI, Inokuchi JI. Ganglioside GM3 synthase deficiency in mouse models and human patients. Int J Mol Sci. 2022;23:5368.
Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, Nagafuku M, et al. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc Natl Acad Sci USA. 2009;106:9483–8.
Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA. 2003;100:3445–9.
Inamori KI, Ito H, Tamura Y, Nitta T, Yang X, Nihei W, et al. Deficient ganglioside synthesis restores responsiveness to leptin and melanocortin signaling in obese KKAy mice. J Lipid Res. 2018;59:1472–81.
Nordstrom V, Willershauser M, Herzer S, Rozman J, von Bohlen Und Halbach O, Meldner S, et al. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLoS Biol. 2013;11:e1001506.
Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA. 2007;104:13678–83.
Yoshikawa M, Go S, Suzuki S, Suzuki A, Katori Y, Morlet T, et al. Ganglioside GM3 is essential for the structural integrity and function of cochlear hair cells. Hum Mol Genet. 2015;24:2796–807.
Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66.
Collins BE, Yang LJ, Mukhopadhyay G, Filbin MT, Kiso M, Hasegawa A, et al. Sialic acid specificity of myelin-associated glycoprotein binding. J Biol Chem. 1997;272:1248–55.
Yang LJ, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, et al. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci USA. 1996;93:814–8.
Novarino G, Fenstermaker AG, Zaki MS, Hofree M, Silhavy JL, Heiberg AD, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science. 2014;343:506–11.
Bharathi SS, Zhang BB, Paul E, Zhang Y, Schmidt AV, Fowler B, et al. GM3 synthase deficiency increases brain glucose metabolism in mice. Mol Genet Metab. 2022;137:342–8.
Tang FL, Wang J, Itokazu Y, Yu RK. Enhanced susceptibility to chemoconvulsant-induced seizures in ganglioside GM3 synthase knockout mice. ASN Neuro. 2020;12:1759091420938175.
Lopez PH, Aja S, Aoki K, Seldin MM, Lei X, Ronnett GV, et al. Mice lacking sialyltransferase ST3Gal-II develop late-onset obesity and insulin resistance. Glycobiology. 2017;27:129–39.
Sturgill ER, Aoki K, Lopez PH, Colacurcio D, Vajn K, Lorenzini I, et al. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology. 2012;22:1289–301.
Yoo SW, Motari MG, Susuki K, Prendergast J, Mountney A, Hurtado A, et al. Sialylation regulates brain structure and function. FASEB J. 2015;29:3040–53.
Takamiya K, Yamamoto A, Furukawa K, Zhao J, Fukumoto S, Yamashiro S, et al. Complex gangliosides are essential in spermatogenesis of mice: possible roles in the transport of testosterone. Proc Natl Acad Sci USA. 1998;95:12147–52.
Liu Y, Su Y, Wiznitzer M, Epifano O, Ladisch S. Ganglioside depletion and EGF responses of human GM3 synthase-deficient fibroblasts. Glycobiology. 2008;18:593–601.
Shevchuk NA, Hathout Y, Epifano O, Su Y, Liu Y, Sutherland M, et al. Alteration of ganglioside synthesis by GM3 synthase knockout in murine embryonic fibroblasts. Biochim Biophys Acta. 2007;1771:1226–34.
Svennerholm L, Bostrom K, Jungbjer B, Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J Neurochem. 1994;63:1802–11.
Hadaczek P, Wu G, Sharma N, Ciesielska A, Bankiewicz K, Davidow AL, et al. GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Exp Neurol. 2015;263:177–89.
Wu G, Lu ZH, Kulkarni N, Ledeen RW. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J Neurosci Res. 2012;90:1997–2008.
Huebecker M, Moloney EB, van der Spoel AC, Priestman DA, Isacson O, Hallett PJ, et al. Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease. Mol Neurodegener. 2019;14:40.
Seyfried TN, Choi H, Chevalier A, Hogan D, Akgoc Z, Schneider JS. Sex-related abnormalities in substantia nigra lipids in Parkinson’s disease. ASN Neuro. 2018;10:1759091418781889.
Schneider JS. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLoS One. 2018;13:e0199189.
Sonnino S. The relationship between depletion of brain GM1 ganglioside and Parkinson’s disease. FEBS Open Bio. 2023;13:1548–57.